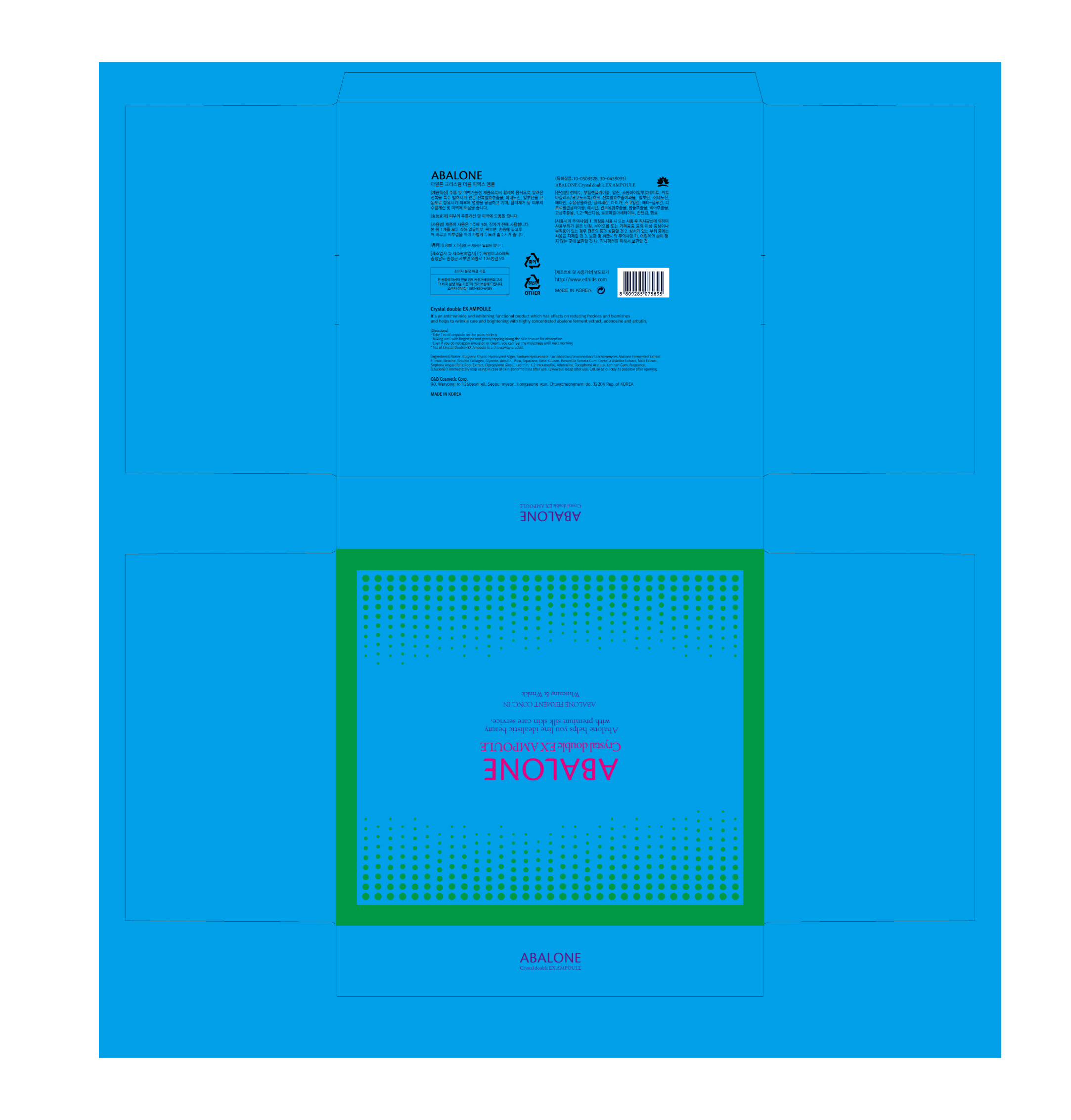
Label: ABALONE CRYSTALDOUBLE EX AMPOULE- arbutin, adenosine liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 60611-0006-1 - Packager: C&BCOSMETIC Co.,Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 10, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTArbutin, Adenosine
-
INACTIVE INGREDIENTWater, Glycerin, Butylene Glycol, Etc.
-
PURPOSESkin Protectant - Anti-Wrinkle & Whitening
-
KEEP OUT OF REACH OF CHILDRENkeep out or reach of the children
-
INDICATIONS & USAGETake 1EA of ampoule on the palm entirely - Mixing well with fingertips and gently tapping along the skin texture for absorption - Even if you do not apply emulsion or cream, you can feel the moistness ...
-
WARNINGS1. Do not use in the following cases(Eczema and scalp wounds) 2.Side Effects - 1)Due to the use of this druf if rash, irritation, itching ...
-
DOSAGE & ADMINISTRATIONfor external use only
-
PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCEProduct Information