Label: ACCLEAN CHLORHEXIDINE GLUCONATE 0.12% ORAL RINSE- chlorhexidine gluconate 0.12% oral rinse liquid
- NDC Code(s): 0116-6720-04
- Packager: Xttrium Laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
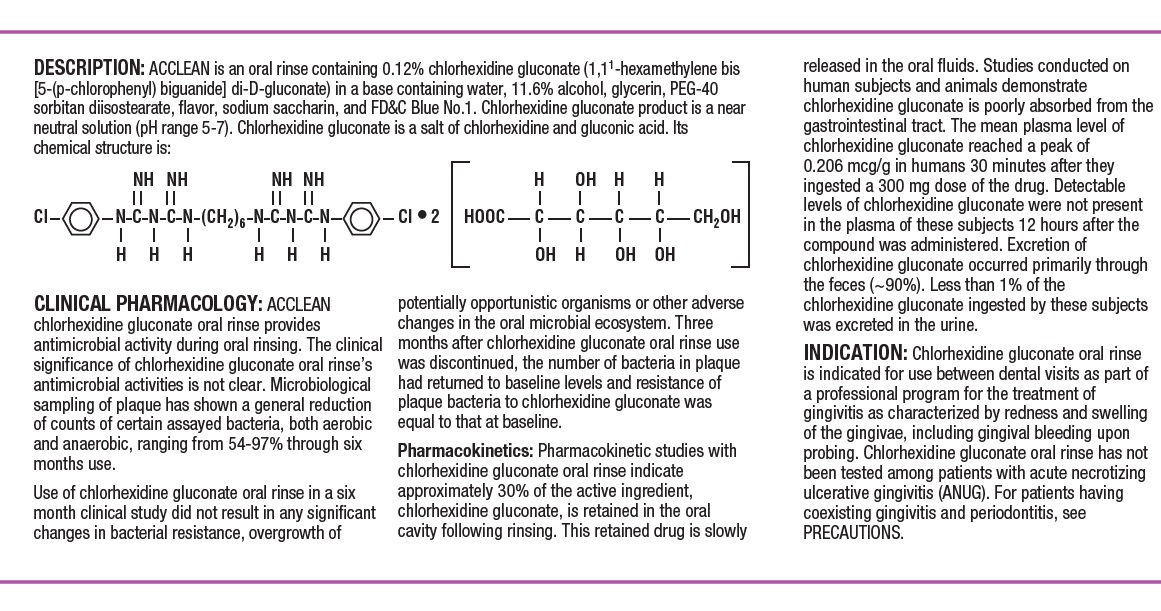
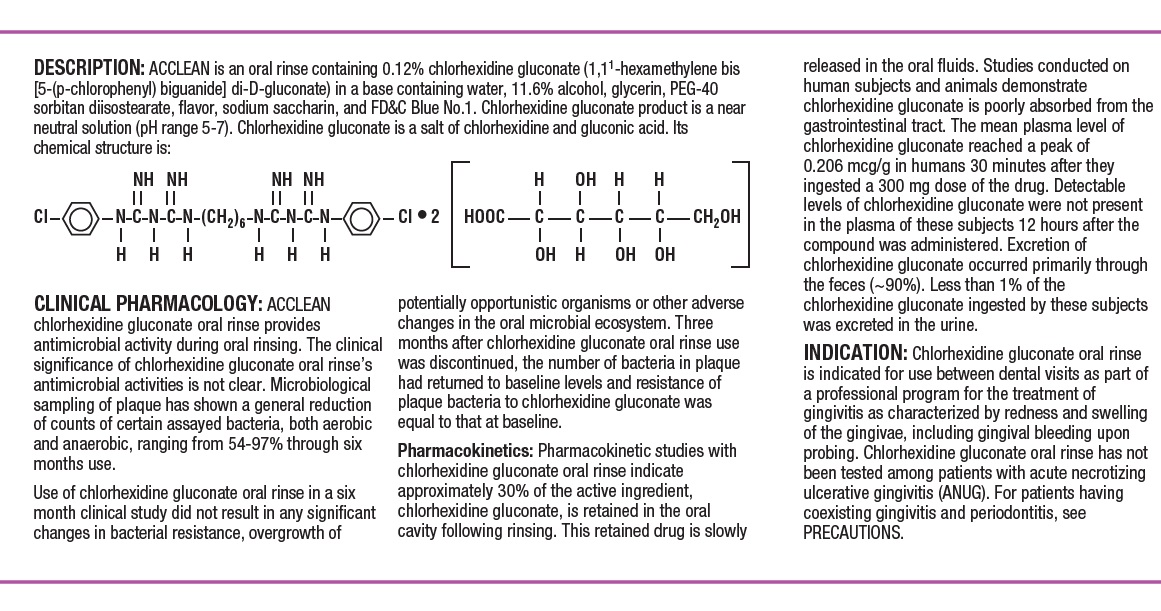
DESCRIPTIONACCLEAN is an oral rinse containing 0.12% chlorhexidine gluconate (1,1’-hexamethylene bis [5-(p-chlorphenyl) biguanide]di-D-gluconate) in a base containing water, 11.6% alcohol, glycerin, PEG-40 ...
-
CLINICAL PHARMACOLOGYACCLEAN chlorhexidine gluconate oral rinse provides antimicrobial activity during oral rinsing. The clinical significance of chlorhexidine gluconate oral rinse’s antimicrobial activities is not ...
-
INDICATIONS & USAGEChlorhexidine gluconate oral rinse is indicated for use between dental visits as part of a professional program for the treatment of gingivitis as characterized by redness and swelling of the ...
-
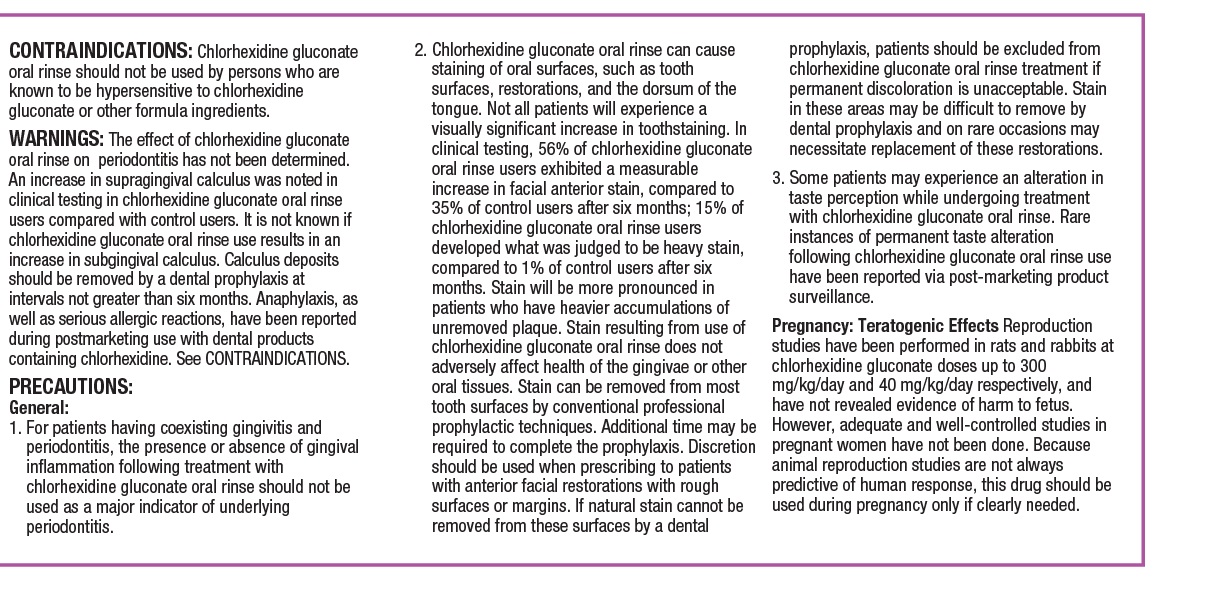
CONTRAINDICATIONSChlorhexidine gluconate oral rinse should not be used by persons who are known to be hypersensitive to chlorhexidine gluconate or other formula ingredients.
-
WARNINGSThe effect of chlorhexidine gluconate oral rinse on periodontitis has not been determined. An increase in supragingival calculus was noted in clinical testing in chlorhexidine gluconate oral rinse ...
-
PRECAUTIONSGeneral: 1. For patients having coexisting gingivitis and periodontitis, the presence or absence of gingival inflammation following treatment with chlorhexidine gluconate oral rinse should not be ...
-
Pregnancy: Teratogenic Effects
Reproduction studies have been performed in rats and rabbits at chlorhexidine gluconate doses up to 300 mg/kg/day and 40 mg/kg/day, respectively, and have not revealed evidence of harm to the ...
-
NURSING MOTHERSIt is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when chlorhexidine gluconate oral rinse is administered to ...
-
PEDIATRIC USEClinical effectiveness and safety of chlorhexidine gluconate oral rinse have not been established in children under the age of 18.
-
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITYIn a drinking water study in rats, carcinogenic effects were not observed at doses up to 38 mg/kg/day. Mutagenic effects were not observed in two mammalian in vivo mutagenesis studies with ...
-
ADVERSE REACTIONSThe most common side effects associated with chlorhexidine gluconate oral rinses are: 1) an increase in staining of teeth and other oral surfaces; 2) an increase in calculus formation; and 3) an ...
-
OVERDOSAGEIngestion of 1 or 2 ounces of chlorhexidine gluconate oral rinse by a small child (~10 kg body weight) might result in gastric distress, including nausea, or signs of alcohol intoxication. Medical ...
-
DOSAGE & ADMINISTRATIONChlorhexidine gluconate oral rinse therapy should be initiated directly following a dental prophylaxis. Patients using chlorhexidine gluconate oral rinse should be reevaluated and given a thorough ...
-
HOW SUPPLIEDChlorhexidine gluconate oral rinse is supplied as a blue liquid in 4-ounce (118 ml) and 1-pint (473 ml) amber plastic bottles with child-resistant dispensing closures. Store at 20°C to 25°C ...
-
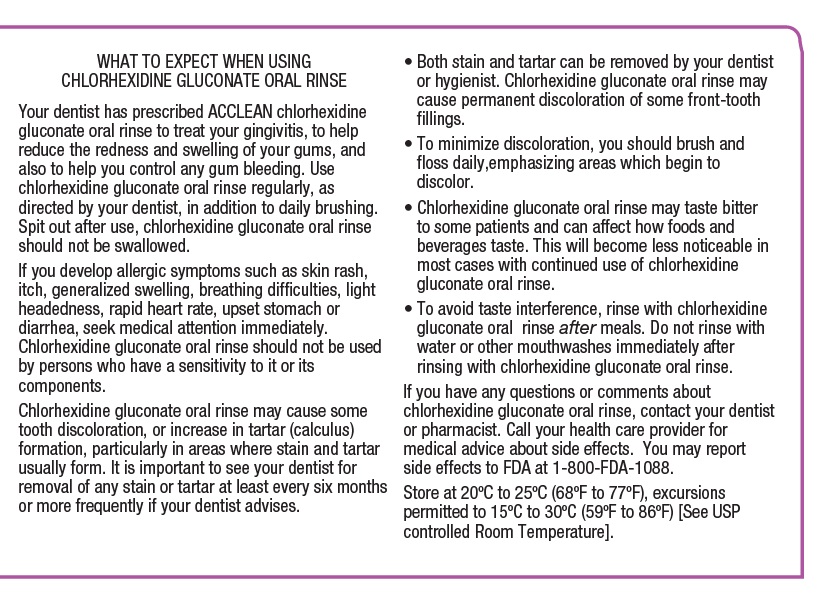
WHAT TO EXPECT WHEN USING CHLORHEXIDINE GLUCONATE ORAL RINSEYour dentist has prescribed ACCLEAN chlorhexidine gluconate oral rinse to treat your gingivitis, to help reduce the redness and swelling of your gums, and also to help you control any gum ...
-
PRINCIPAL DISPLAY PANELNDC 0404-6720-04 - REF 112-6720 - ACCLEAN ® Chlorhexidine Gluconate - 0.12% Oral Rinse, USP - Anti-Microbial Rinse, Mint - DIRECTIONS FOR USE: Swish 1 tablespoon (15 ml) in mouth undiluted for 30 seconds ...
-
INGREDIENTS AND APPEARANCEProduct Information