Label: MESNEX- mesna injection, solution
- NDC Code(s): 0338-1305-01, 0338-1305-03
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 17, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MESNEX safely and effectively. See full prescribing information for MESNEX. MESNEX (mesna) tablets, for oral use - MESNEX (mesna ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMESNEX is indicated as a prophylactic agent in reducing the incidence of ifosfamide-induced hemorrhagic cystitis. Limitation of Use: MESNEX is not indicated to reduce the risk of hematuria due ...
-
2 DOSAGE AND ADMINISTRATION2.1 Intravenous Dosing - MESNEX may be given on a fractionated dosing schedule of three bolus intravenous injections as outlined below. MESNEX injection is given as intravenous bolus injections ...
-
3 DOSAGE FORMS AND STRENGTHS• MESNEX (mesna) injection: 1 g Multidose Vial, 100 mg/mL - • MESNEX (mesna) tablets: 400 mg film-coated tablets with functional score
-
4 CONTRAINDICATIONSMESNEX is contraindicated in patients known to be hypersensitive to mesna or to any of the excipients [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - MESNEX may cause systemic hypersensitivity reactions, including anaphylaxis. These reactions may include fever, cardiovascular symptoms (hypotension ...
-
6 ADVERSE REACTIONSThe following are discussed in more detail in other sections of the labeling. • Hypersensitivity Reactions [see Warnings and Precautions (5.1)] • Dermatological Toxicity [see Warnings and ...
-
7 DRUG INTERACTIONSNo clinical drug interaction studies have been conducted with MESNEX.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - MESNEX is used in combination with ifosfamide or other cytotoxic agents. Ifosfamide can cause fetal harm when administered to a pregnant woman. Refer to the ...
-
10 OVERDOSAGEThere is no known antidote for MESNEX. In a clinical trial, 11 patients received intravenous MESNEX 10 mg/kg to 66 mg/kg per day for 3 to 5 days. Patients also received ifosfamide or ...
-
11 DESCRIPTIONMESNEX (mesna) is a detoxifying agent to inhibit the hemorrhagic cystitis induced by ifosfamide. The active ingredient, mesna, is a synthetic sulfhydryl compound designated as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Mesna reacts chemically with the urotoxic ifosfamide metabolites, acrolein and 4-hydroxy-ifosfamide, resulting in their detoxification. The first step in the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No long-term studies in animals have been performed to evaluate the carcinogenic potential of mesna. Mesna was not genotoxic in the in ...
-
14 CLINICAL STUDIES14.1 Intravenous MESNEX - Hemorrhagic cystitis produced by ifosfamide is dose dependent (Table 4). At a dose of 1.2 g/m2 ifosfamide administered daily for 5 days, 16 to 26% of the patients who ...
-
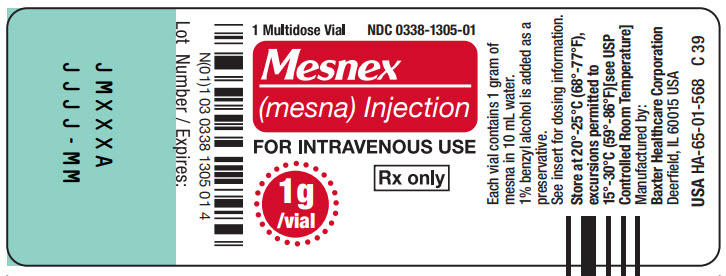
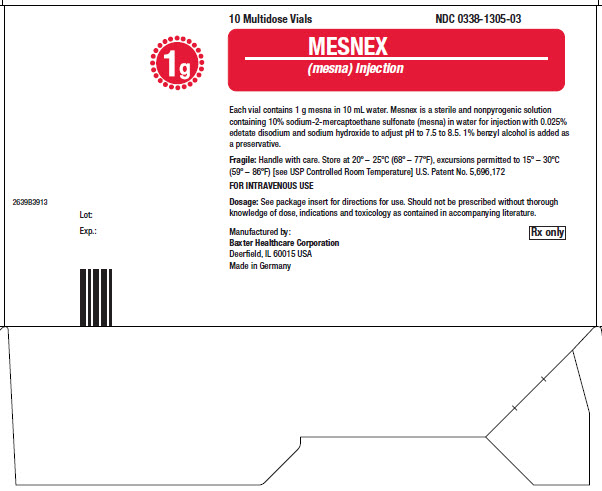
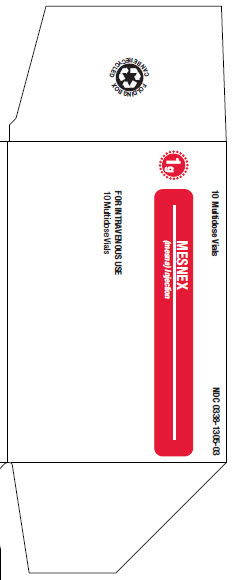
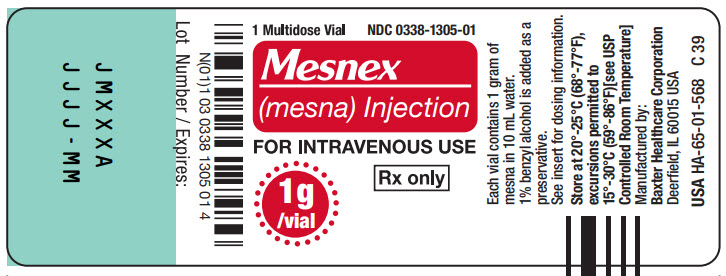
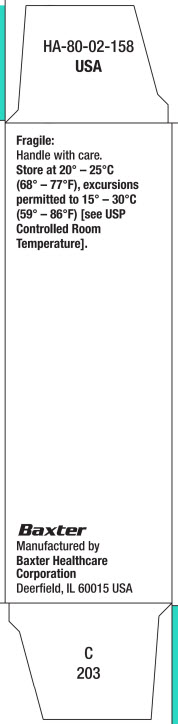
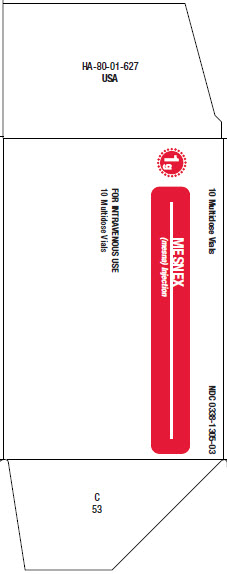
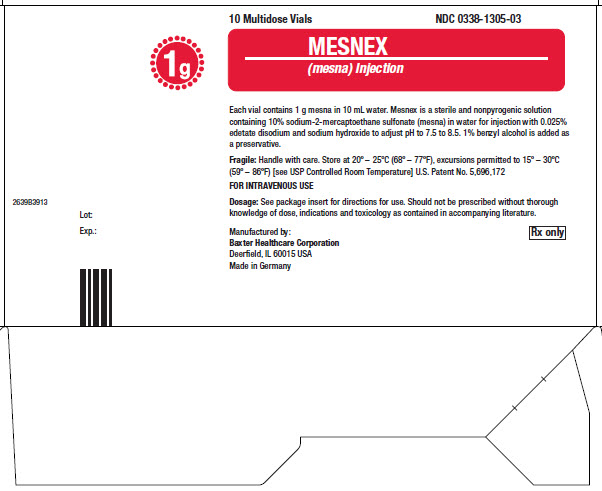
16 HOW SUPPLIED/STORAGE AND HANDLINGMESNEX (mesna) injection 100 mg/mL - • NDC 0338-1305-01 1 g Multidose Vial, Box of 1 vial of 10 mL - • NDC 0338-1305-03 1 g Multidose Vial, Box of 10 vials of 10 mL - Store at 20°C to 25°C (68°F to ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Patient Information). Hypersensitivity - • Advise the patient to discontinue MESNEX and seek immediate medical attention if any signs or symptoms of a ...
-
SPL UNCLASSIFIED SECTIONBaxter Logo - MESNEX (mesna) injection manufactured by: MESNEX (mesna) tablets manufactured for: Baxter Healthcare Corporation - Deerfield, IL 60015 USA - For Product Inquiry 1800 ANA DRUG ...
-
PATIENT MEDICATION INFORMATIONPatient Information - MESNEX (MES-nex) (mesna) tablets - MESNEX (MES-nex) (mesna) injection - What is the most important information I should know about MESNEX? MESNEX can cause ...
-
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL1g Multidose Vial - 1g Multidose Vial - 1 Multidose Vial NDC 0338-1305-01 - Mesnex - (mesna) Injection - FOR INTRAVENOUS USE - 1g/vial Rx only - N (01) 1 03 0338 1305 01 4 - Lot Number ...
-
INGREDIENTS AND APPEARANCEProduct Information