Label: ARIKAYCE- amikacin suspension
- NDC Code(s): 71558-590-28
- Packager: Insmed Incorporated
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 17, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ARIKAYCE safely and effectively. See full prescribing information for ARIKAYCE. ARIKAYCE® (amikacin liposome inhalation ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: RISK OF INCREASED RESPIRATORY ADVERSE REACTIONS
ARIKAYCE has been associated with an increased risk of respiratory adverse reactions including, hypersensitivity pneumonitis, hemoptysis, bronchospasm, exacerbation of underlying pulmonary disease that have led to hospitalizations in some cases [see Warnings and Precautions (5.1, 5.2, 5.3, 5.4)].
Close -
1 INDICATIONS AND USAGELIMITED POPULATION: ARIKAYCE® is indicated in adults, who have limited or no alternative treatment options, for the treatment of Mycobacterium avium complex (MAC) lung disease as part of a ...
-
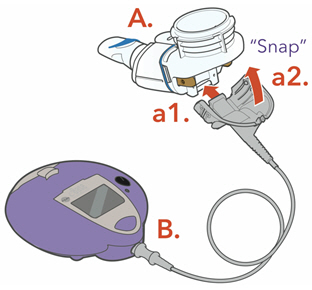
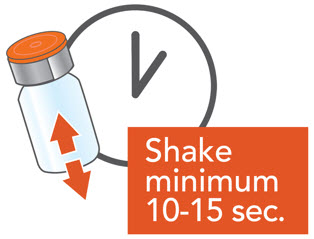
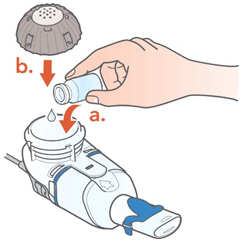
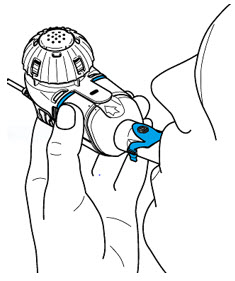
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - ARIKAYCE is for oral inhalation use only. Administer by nebulization only with the Lamira® Nebulizer System. Refer to the Instructions for Use for full ...
-
3 DOSAGE FORMS AND STRENGTHSARIKAYCE is supplied as a sterile, white, milky, aqueous, liposome suspension for oral inhalation in a unit-dose glass vial containing amikacin 590 mg/8.4 mL (equivalent to amikacin sulfate 788 ...
-
4 CONTRAINDICATIONSARIKAYCE is contraindicated in patients with a known hypersensitivity to any aminoglycoside.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Pneumonitis - Hypersensitivity pneumonitis has been reported with the use of ARIKAYCE in the clinical trials. Hypersensitivity pneumonitis (reported as allergic alveolitis ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described in greater detail in other sections of labeling: Hypersensitivity pneumonitis [see Boxed Warning and Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Drugs with Neurotoxic, Nephrotoxic, or Ototoxic Potential - Avoid concomitant use of ARIKAYCE with medications associated with neurotoxicity, nephrotoxicity, and ototoxicity. 7.2 ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no data on ARIKAYCE use in pregnant women to evaluate for any drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal ...
-
10 OVERDOSAGEAdverse reactions specifically associated with overdose of ARIKAYCE have not been identified. Acute toxicity should be treated with immediate withdrawal of ARIKAYCE, and baseline tests of renal ...
-
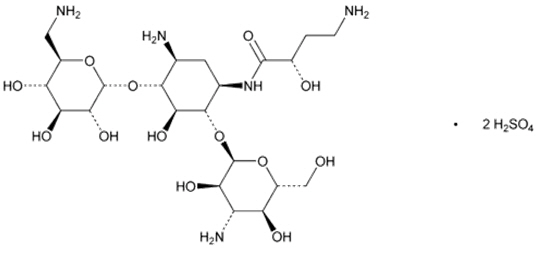
11 DESCRIPTIONThe active ingredient in ARIKAYCE (amikacin liposome inhalation suspension) is amikacin sulfate USP, an aminoglycoside antibacterial. Its chemical name is D-Streptamine ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - ARIKAYCE is an antibacterial drug [see Microbiology (12.4)]. 12.2 Pharmacodynamics - ARIKAYCE exposure-response relationships and the time course of pharmacodynamic ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 2-year inhalation carcinogenicity study, rats were exposed to ARIKAYCE for 15-25, 50-70, or 155-170 minutes per day for 96-104 ...
-
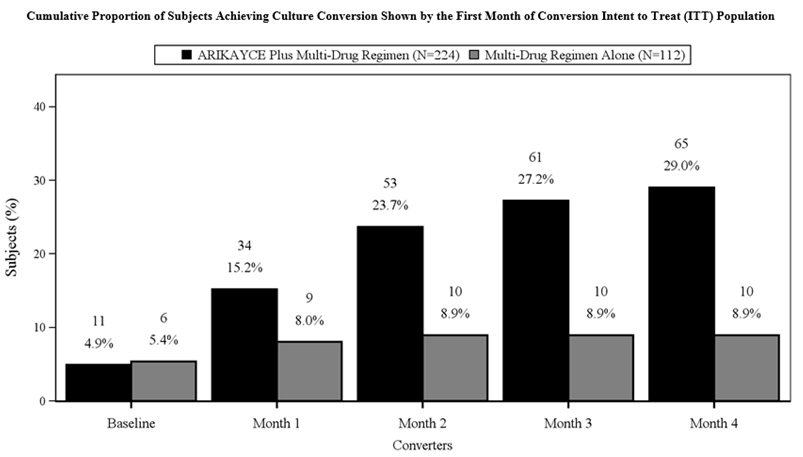
14 CLINICAL STUDIESTrial 1 (NCT#02344004) was an open-label, randomized (2:1), multi-center trial in patients with refractory Mycobacterium avium complex (MAC) lung disease as confirmed by at least 2 sputum culture ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - ARIKAYCE (amikacin liposome inhalation suspension), 590 mg/8.4 mL, is supplied in a sterile, unit-dose 10-mL glass vial. The product is dispensed as a 28-vial kit. Each carton ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide and Patient Instructions for Use). Important Instructions for Administration of ARIKAYCE - Instruct patients to ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Insmed Incorporated - Bridgewater, NJ 08807 - Insmed® and ARIKAYCE® are trademarks of Insmed Incorporated. Lamira® is a trademark of PARI Pharma GmbH. © 2018 Insmed Incorporated. All ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug AdministrationIssued 01/2025 - MEDICATION GUIDE - ARIKAYCE (ar' i kase) LIMITED POPULATION - (amikacin liposome inhalation ...
-
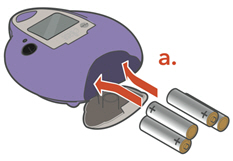
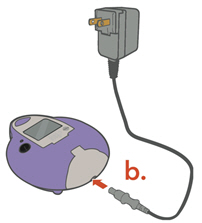
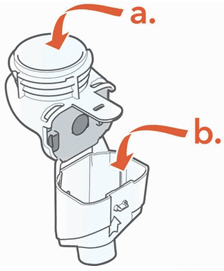
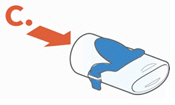
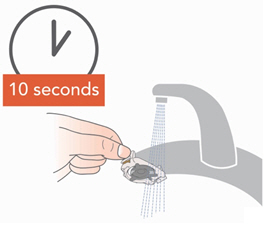
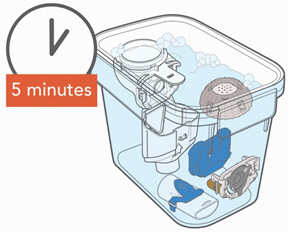
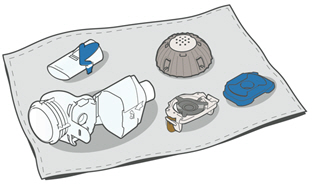
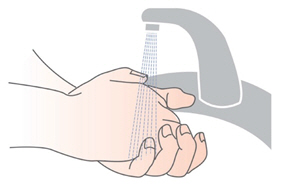
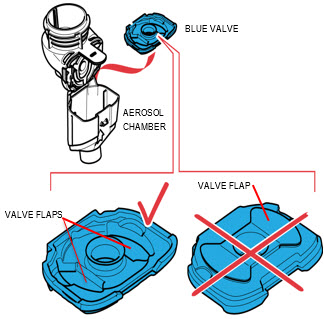
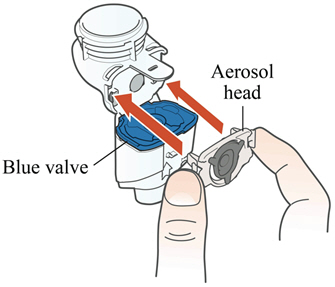
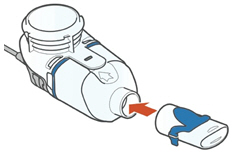
Instructions for UseARIKAYCE® LIMITED POPULATION - (amikacin liposome inhalation suspension) For oral inhalation use - Lamira® Nebulizer System - Before using your Lamira Nebulizer System, be sure you read and ...
-
PRINCIPAL DISPLAY PANEL - 8.4 mL Vial Carton1-833-LIGHT-00 - (1-833-544-4800) Contains 28 sterile unit-dose vials - Each vial contains amikacin 590 mg/8.4 mL - (equivalent to amikacin sulfate 788 mg/8.4 mL) For oral inhalation ...
-
INGREDIENTS AND APPEARANCEProduct Information