Label: PENTAMIDINE ISETHIONATE inhalant
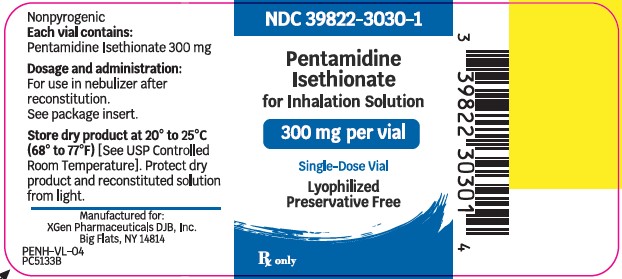
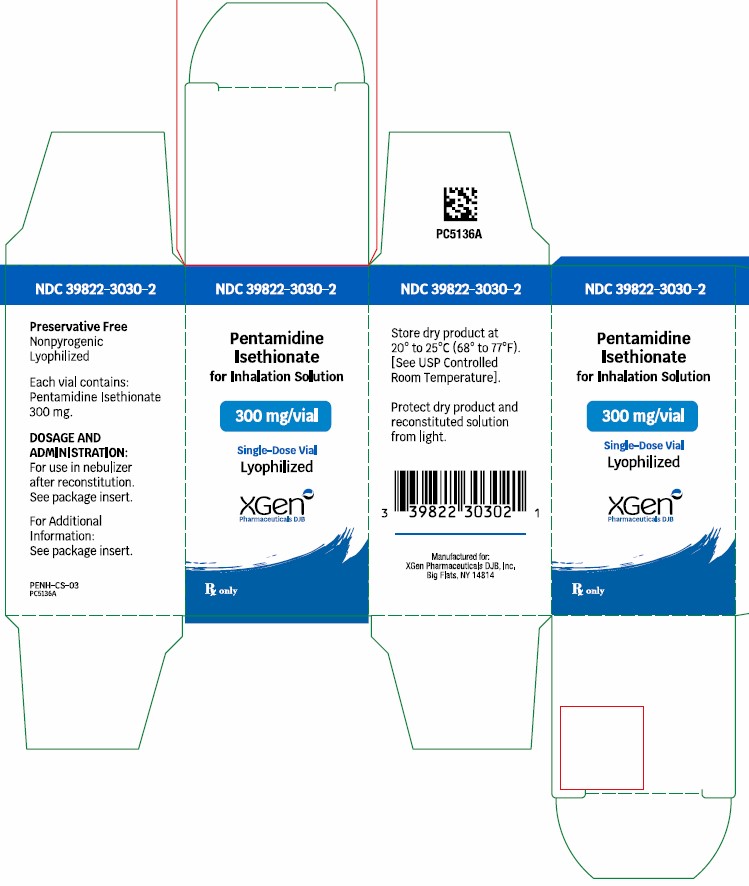
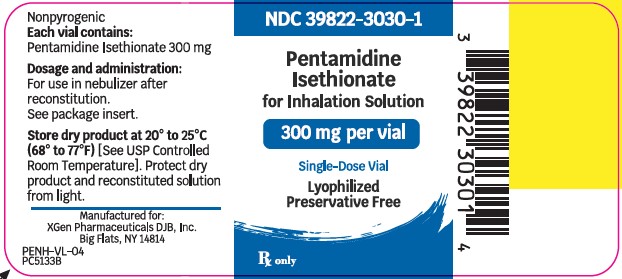
- NDC Code(s): 39822-3030-1, 39822-3030-2
- Packager: XGen Pharmaceuticals DJB, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFor Oral Inhalation Only
-
DESCRIPTION
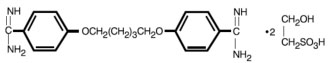
Pentamidine Isethionate for inhalation, an antifungal agent, is a nonpyrogenic lyophilized product. After reconstitution with Sterile Water for Injection, USP, Pentamidine Isethionate is ...
-
CLINICAL PHARMACOLOGY
Microbiology - Mechanism of Action - Studies suggest that the pentamidine isethionate interferes with microbial nuclear metabolism by inhibition of DNA, RNA, phospholipid and protein ...
-
INDICATIONS AND USAGE
Pentamidine Isethionate is indicated for the prevention of - Pneumocystis jiroveci pneumonia (PJP) in high-risk, HIV-infected patients defined by one or both of the following criteria: i. a ...
-
CONTRAINDICATIONS
Pentamidine Isethionate is contraindicated in patients with a history of an anaphylactic reaction to inhaled or parenteral pentamidine isethionate.
-
WARNINGS
The potential for development of acute PJP still exists in patients receiving Pentamidine Isethionate prophylaxis. Therefore, any patient with symptoms suggestive of the presence of a pulmonary ...
-
PRECAUTIONS
IMPORTANT: DO NOT MIX THE PENTAMIDINE ISETHIONATE SOLUTION WITH ANY OTHER DRUGS. DO NOT USE THE RESPIRGARD® II NEBULIZER TO ADMINISTER A BRONCHODILATOR. (See - DOSAGE AND ADMINISTRATION) ...
-
ADVERSE REACTIONS
The most frequently reported unsolicited adverse events (1 to 5%) in clinical trials, regardless of their relation to Pentamidine Isethionate therapy were as follows (n=931): Body as a Whole ...
-
OVERDOSAGE
Overdosage has not been reported with Pentamidine Isethionate. The symptoms and signs of overdosage are not known. A serious overdosage, to the point of producing systemic drug levels similar to ...
-
DOSAGE AND ADMINISTRATION
IMPORTANT: PENTAMIDINE ISETHIONATE MUST BE DISSOLVED ONLY IN STERILE WATER FOR INJECTION, USP. DO NOT USE SALINE SOLUTION FOR RECONSTITUTION BECAUSE THE DRUG WILL PRECIPITATE. DO NOT MIX THE ...
-
HOW SUPPLIED
Pentamidine Isethionate 300 mg lyophilized product is supplied as: Single-dose glass vial (NDC 39822-3030-01), individually packaged as 1 vial per carton (NDC 39822-3030-02) Store dry ...
-
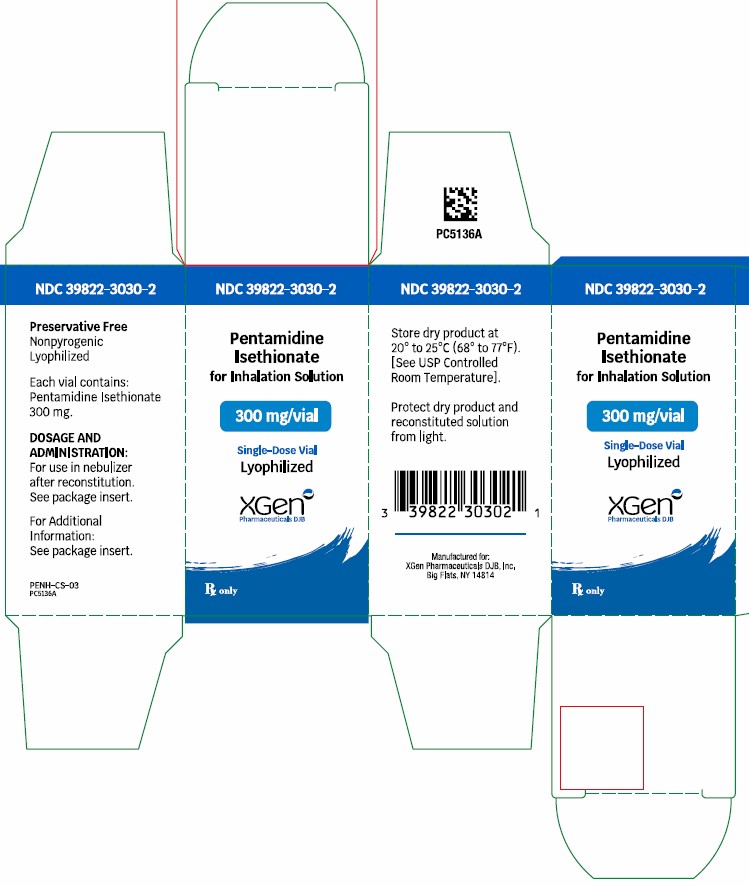
PRINCIPLE PACKAGE DISPLAYPACKAGE LABEL – PRINCIPAL DISPLAY – Pentamidine Isethionate 300 mg Single Dose Vial Label - NDC 39822-3030-1 - Pentamidine Isethionate - 300 mg - Lyophilized - For Inhalation ...
-
INGREDIENTS AND APPEARANCEProduct Information