Label: SULFASALAZINE tablet
- NDC Code(s): 59762-5000-1, 59762-5000-2, 59762-5000-5, 59762-5000-6
- Packager: Mylan Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated May 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTIONSulfasalazine tablets contain sulfasalazine, 500 mg, for oral administration. Therapeutic Classification: Anti-inflammatory agent. Chemical Designation: 5-([p-(2-pyridylsulfamoyl)phenyl]azo ...
-
CLINICAL PHARMACOLOGYPharmacodynamics - The mode of action of sulfasalazine (SSZ) or its metabolites, 5-aminosalicylic acid (5-ASA) and sulfapyridine (SP), may be related to the anti-inflammatory and/or ...
-
INDICATIONS AND USAGESulfasalazine tablets are indicated: a) in the treatment of mild to moderate ulcerative colitis, and as adjunctive therapy in severe ulcerative colitis; and - b) for the prolongation of the ...
-
CONTRAINDICATIONSSulfasalazine tablets are contraindicated in: Patients with intestinal or urinary obstruction, Patients with porphyria as sulfonamides have been reported to precipitate an acute ...
-
WARNINGSHepatic, Renal, and Hematologic Toxicity or Other Conditions - Only after critical appraisal should Sulfasalazine tablets be given to patients with hepatic or renal damage or blood dyscrasias ...
-
PRECAUTIONSGeneral: Sulfasalazine tablets should be given with caution to patients with severe allergy or bronchial asthma. Adequate fluid intake must be maintained in order to prevent crystalluria and ...
-
ADVERSE REACTIONSThe most common adverse reactions associated with sulfasalazine are anorexia, headache, nausea, vomiting, gastric distress, and apparently reversible oligospermia. These occur in about one-third ...
-
DRUG ABUSE AND DEPENDENCENone reported.
-
OVERDOSAGEThere is evidence that the incidence and severity of toxicity following overdosage are directly related to the total serum sulfapyridine concentration. Symptoms of overdosage may include nausea ...
-
DOSAGE AND ADMINISTRATIONThe dosage of sulfasalazine tablets should be adjusted to each individual's response and tolerance. Initial Therapy: Adults: 3 to 4 g daily in evenly divided doses with dosage intervals not ...
-
HOW SUPPLIEDSulfasalazine tablets, 500 mg, are round, gold-colored, scored tablets, monogrammed "G500". They are available in the following package sizes: Bottle of 100(with carton) NDC ...
-
REFERENCES1. Mogadam M, et al. Pregnancy in inflammatory bowel disease: effect of sulfasalazine and corticosteroids on fetal outcome. Gastroenterology 1981; 80:72–6. 2. Kaufman DW, editor. Birth defects ...
-
SPL UNCLASSIFIED SECTIONLAB-0243-18.0 - Revised: 02/2025
-
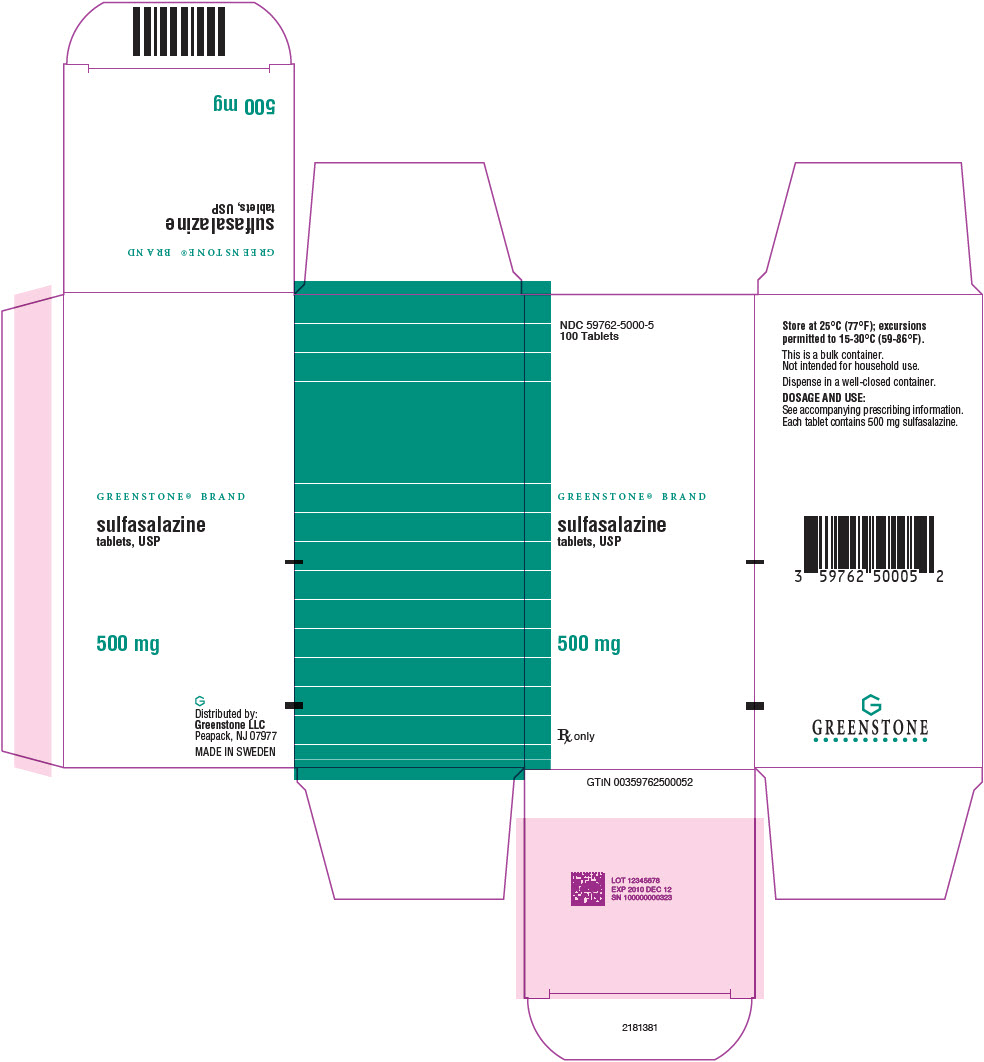
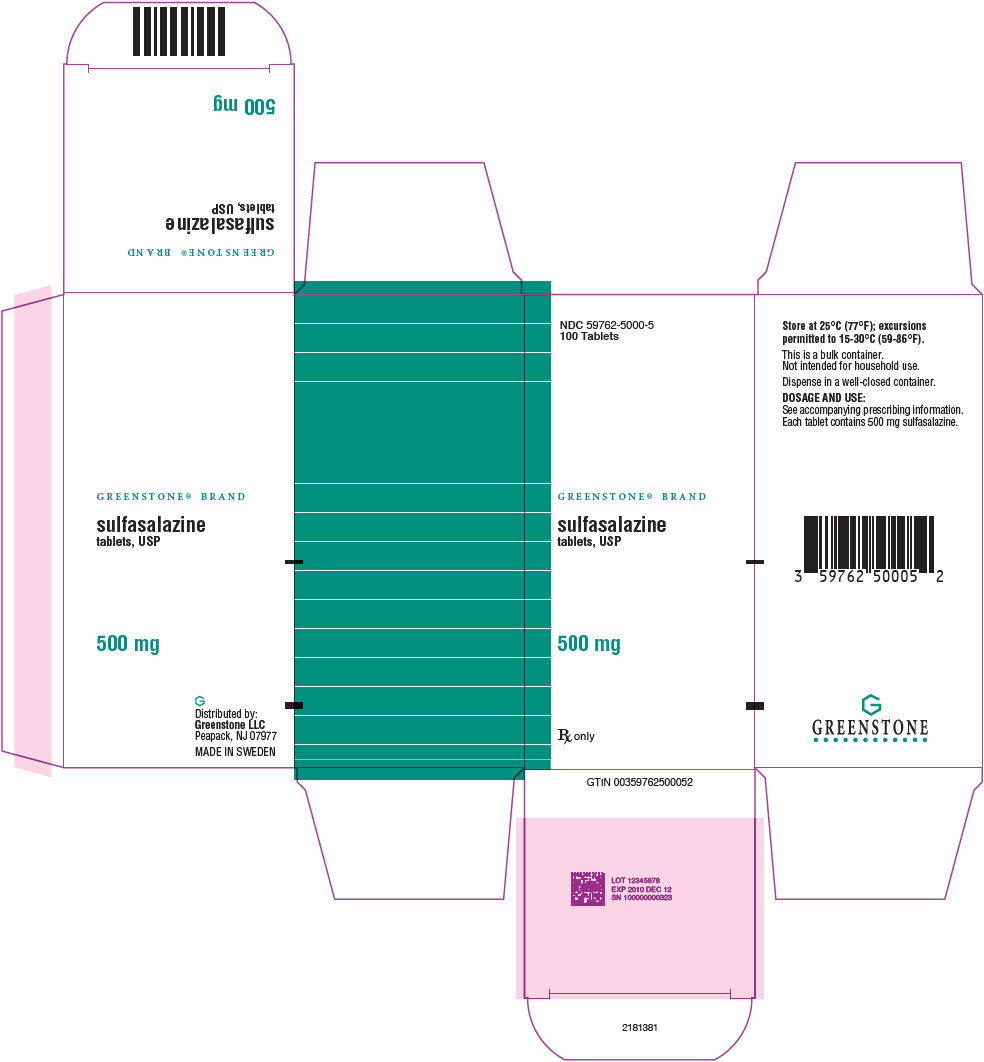
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle LabelNDC 59762-5000-1 - 100 Tablets - GREENSTONE® BRAND - sulfasalazine - tablets, USP - 500 mg - Rx only
-
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Label - 59762-5000-5NDC 59762-5000-5 - 100 Tablets - GREENSTONE® BRAND - sulfasalazine - tablets, USP - 500 mg - Rx only
-
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Carton - 59762-5000-5NDC 59762-5000-5 - 100 Tablets - GREENSTONE® BRAND - sulfasalazine - tablets, USP - 500 mg - Rx only
-
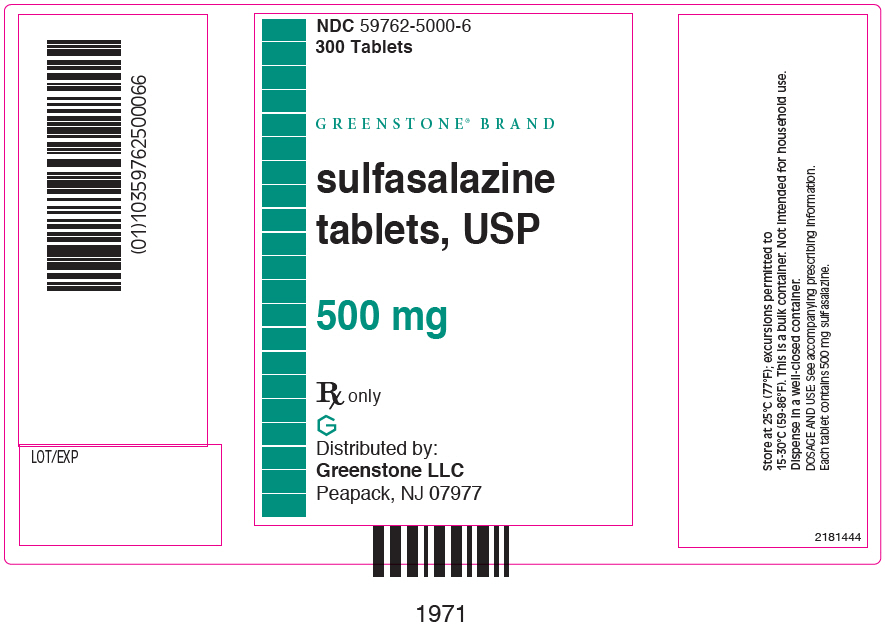
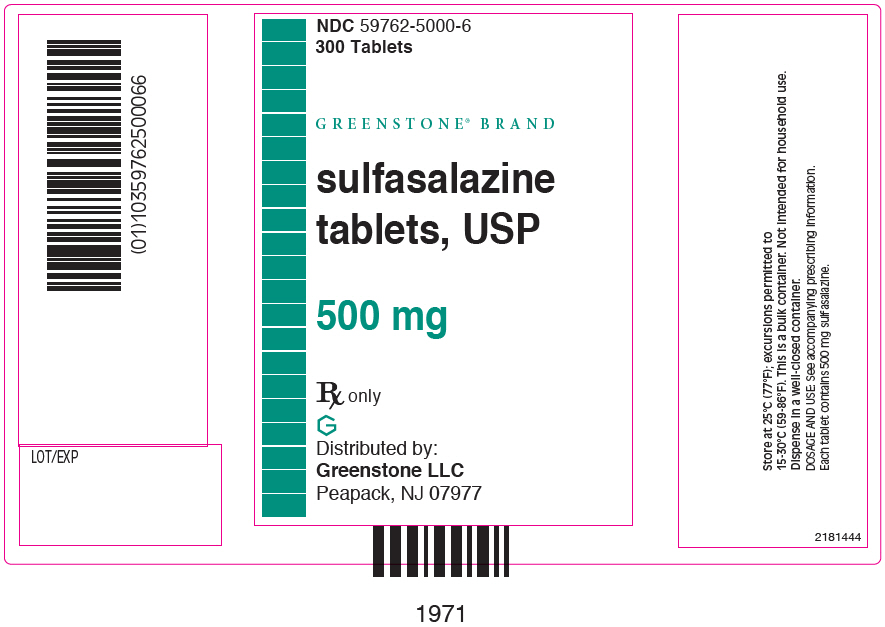
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Label - 59762-5000-6NDC 59762-5000-6 - 300 Tablets - GREENSTONE® BRAND - sulfasalazine - tablets, USP - 500 mg - Rx only - Distributed by: Greenstone LLC - Morgantown, WV 26505 U.S.A.
-
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Carton - 59762-5000-6NDC 59762-5000-6 - 300 Tablets - GREENSTONE® BRAND - sulfasalazine - tablets, USP - 500 mg - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information