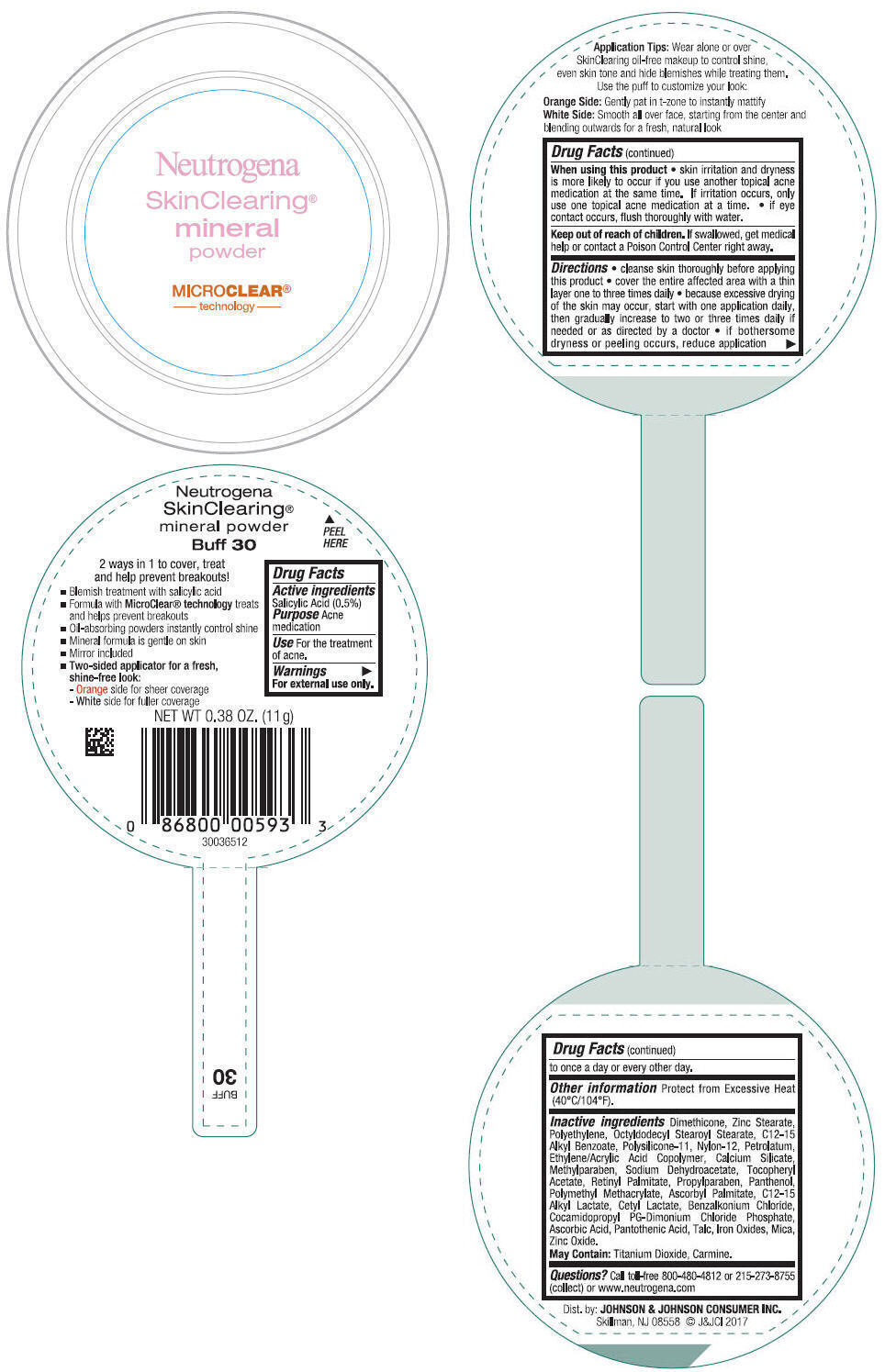
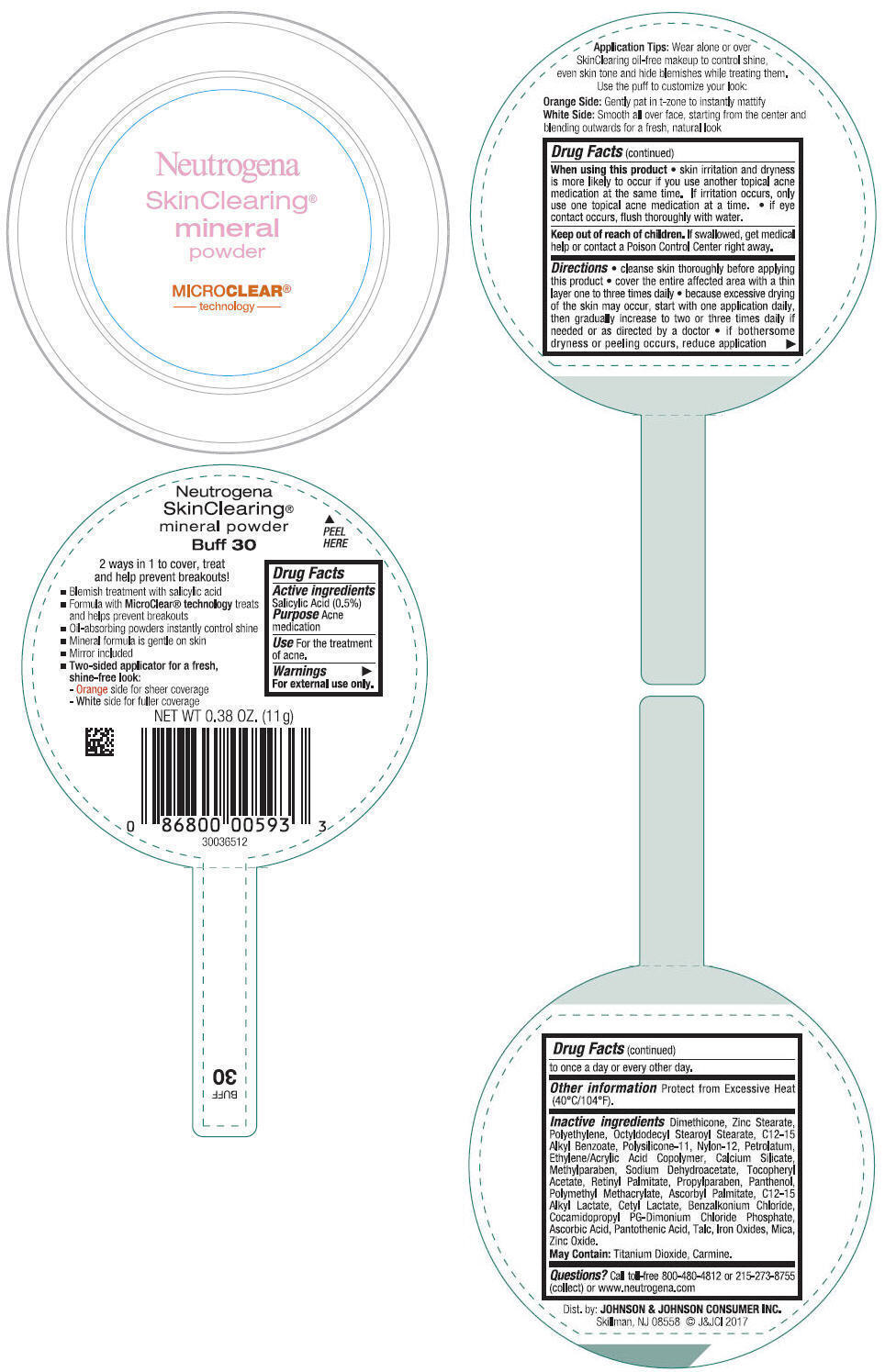
Label: NEUTROGENA SKINCLEARING MINERAL - BUFF- salicylic acid powder
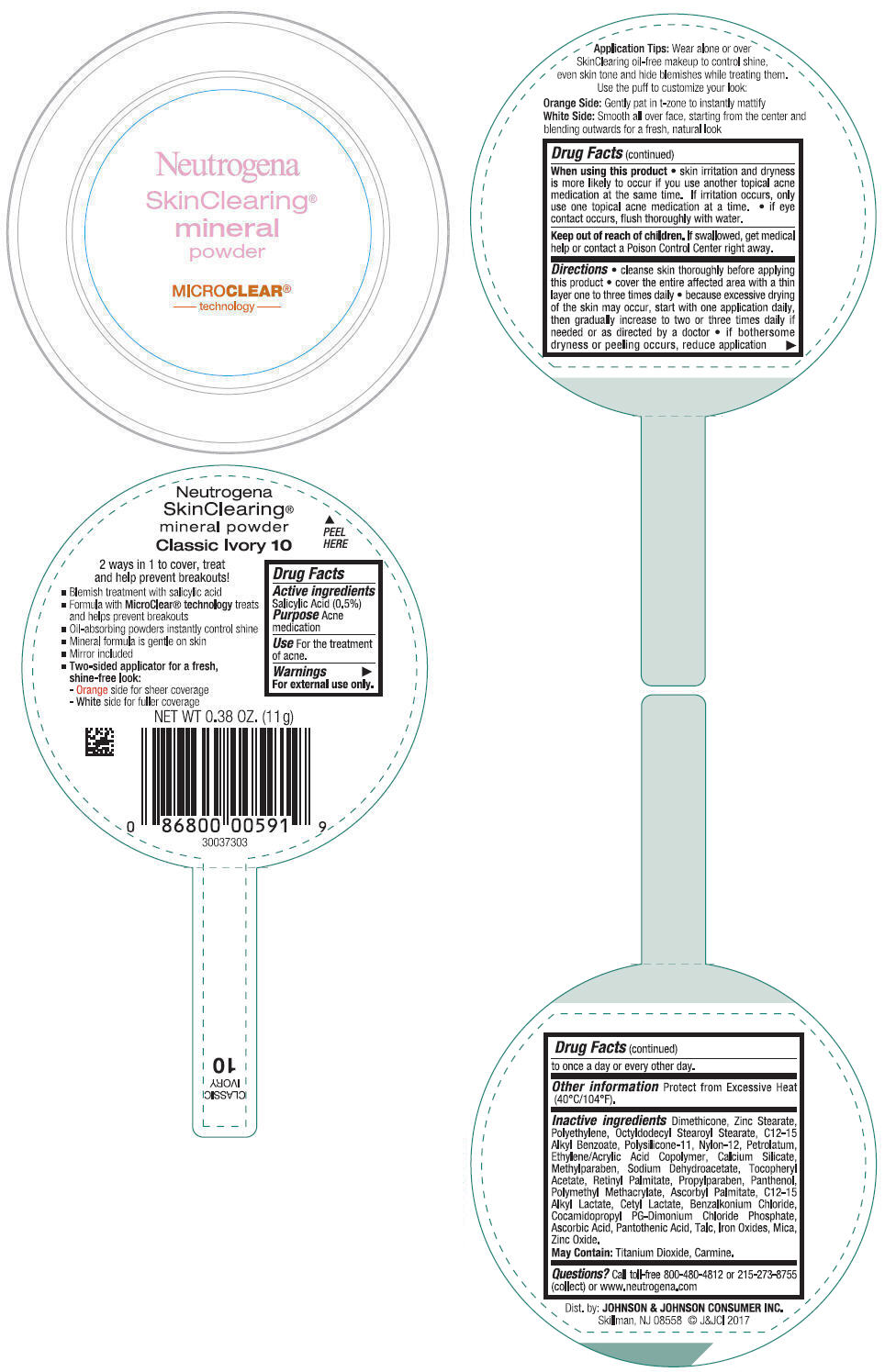
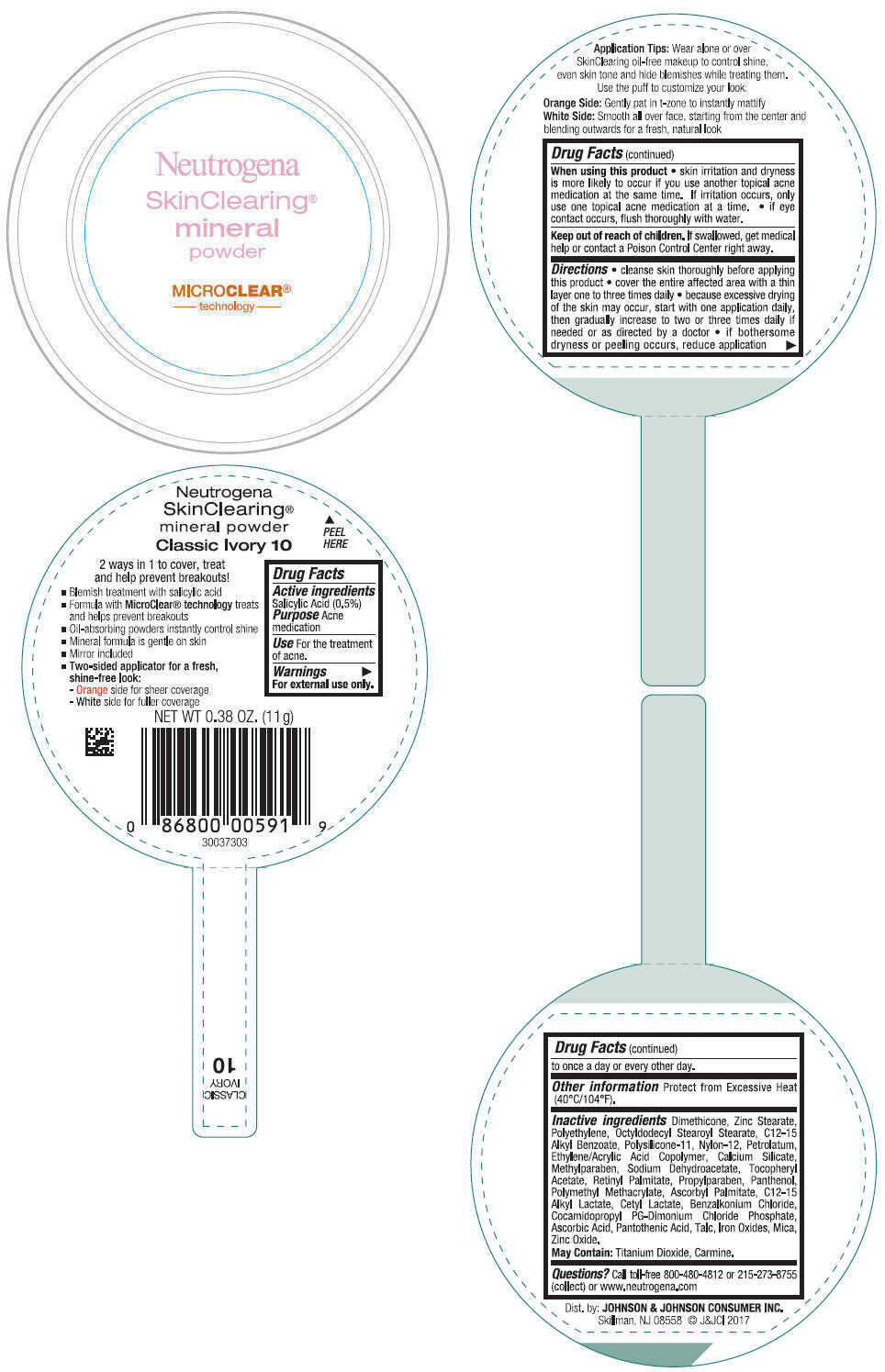
NEUTROGENA SKINCLEARING MINERAL - CLASSIC IVORY- salicylic acid powder
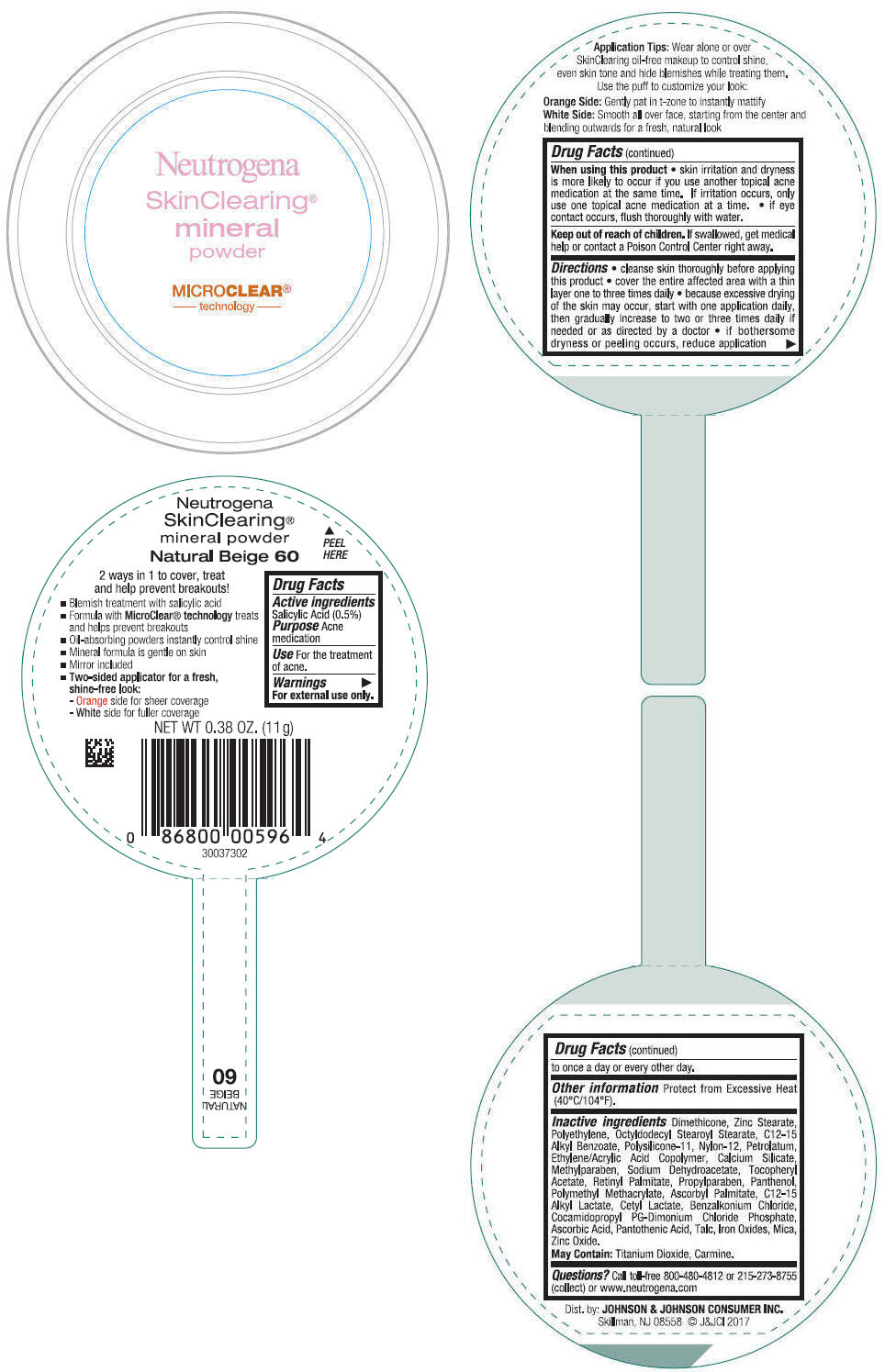
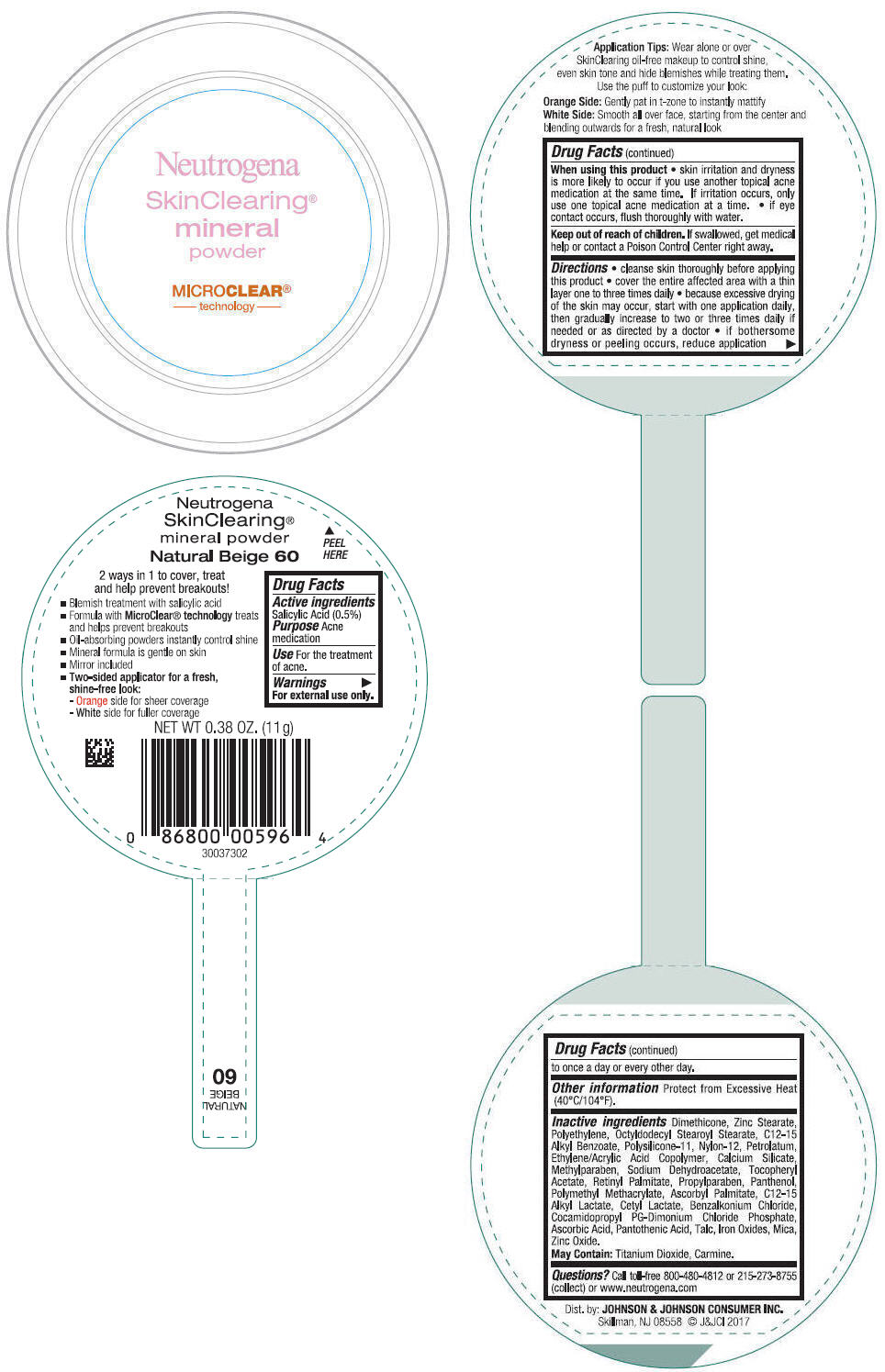
NEUTROGENA SKINCLEARING MINERAL - NATURAL BEIGE- salicylic acid powder
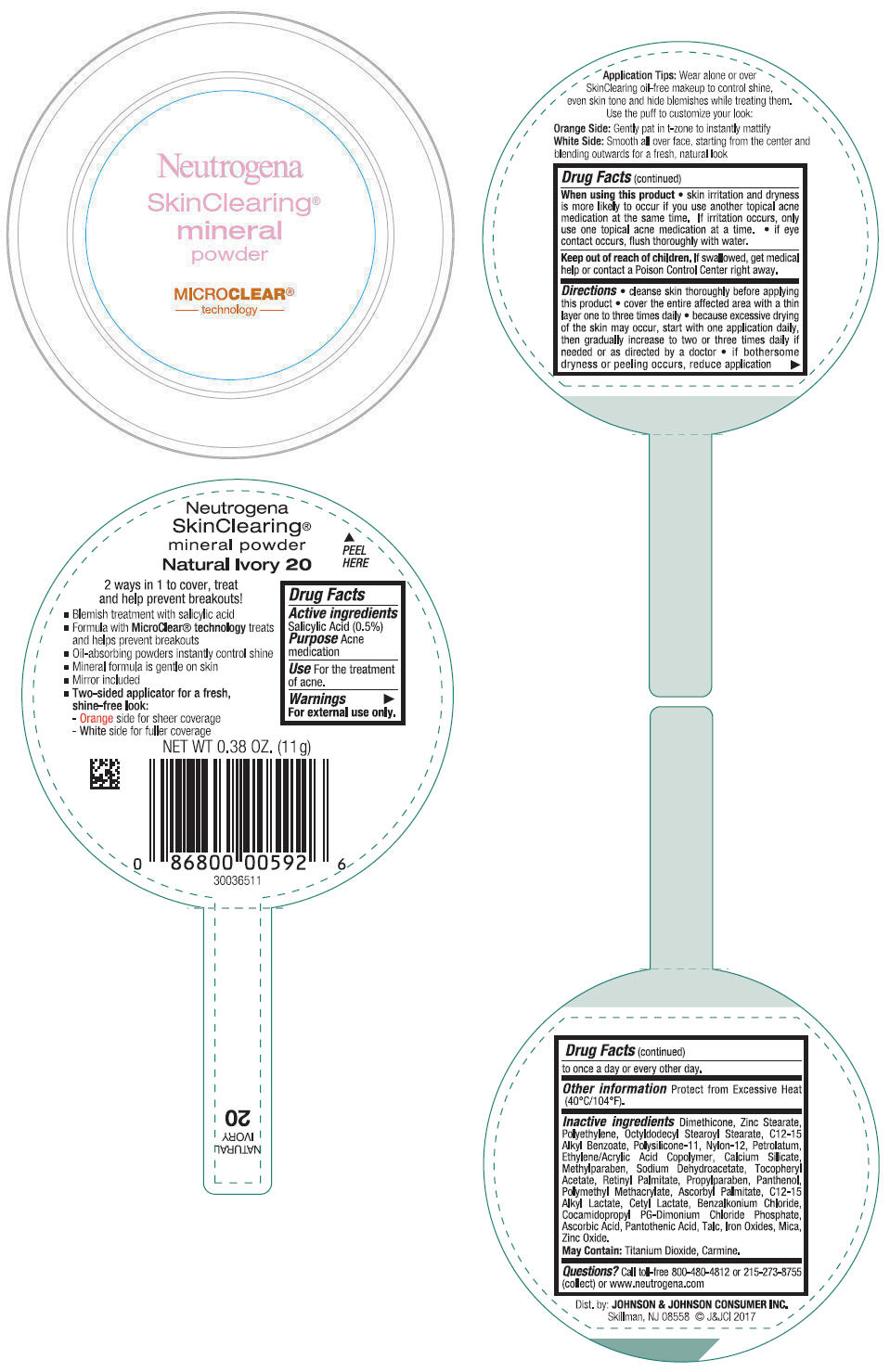
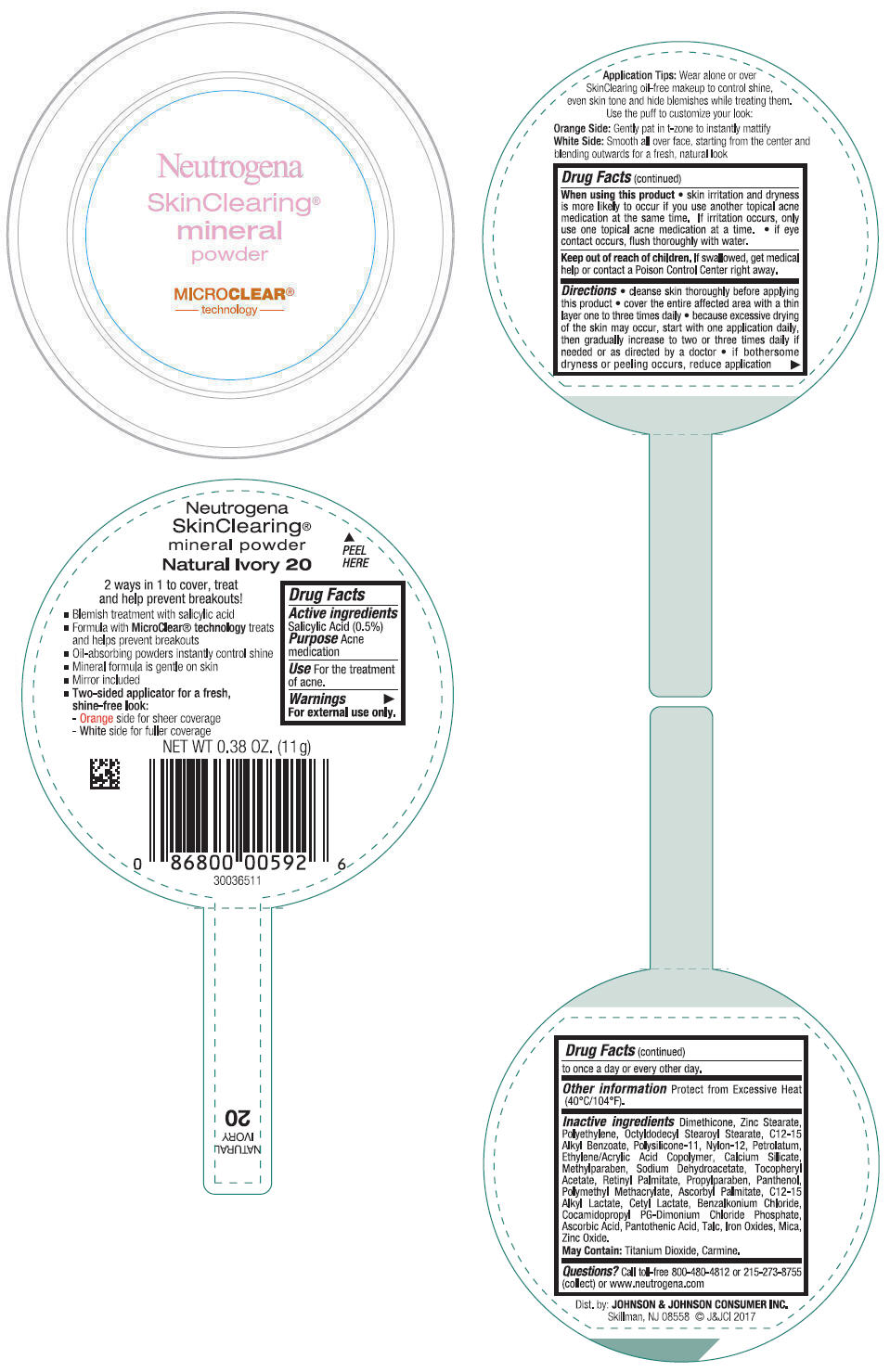
NEUTROGENA SKINCLEARING MINERAL - NATURAL IVORY- salicylic acid powder
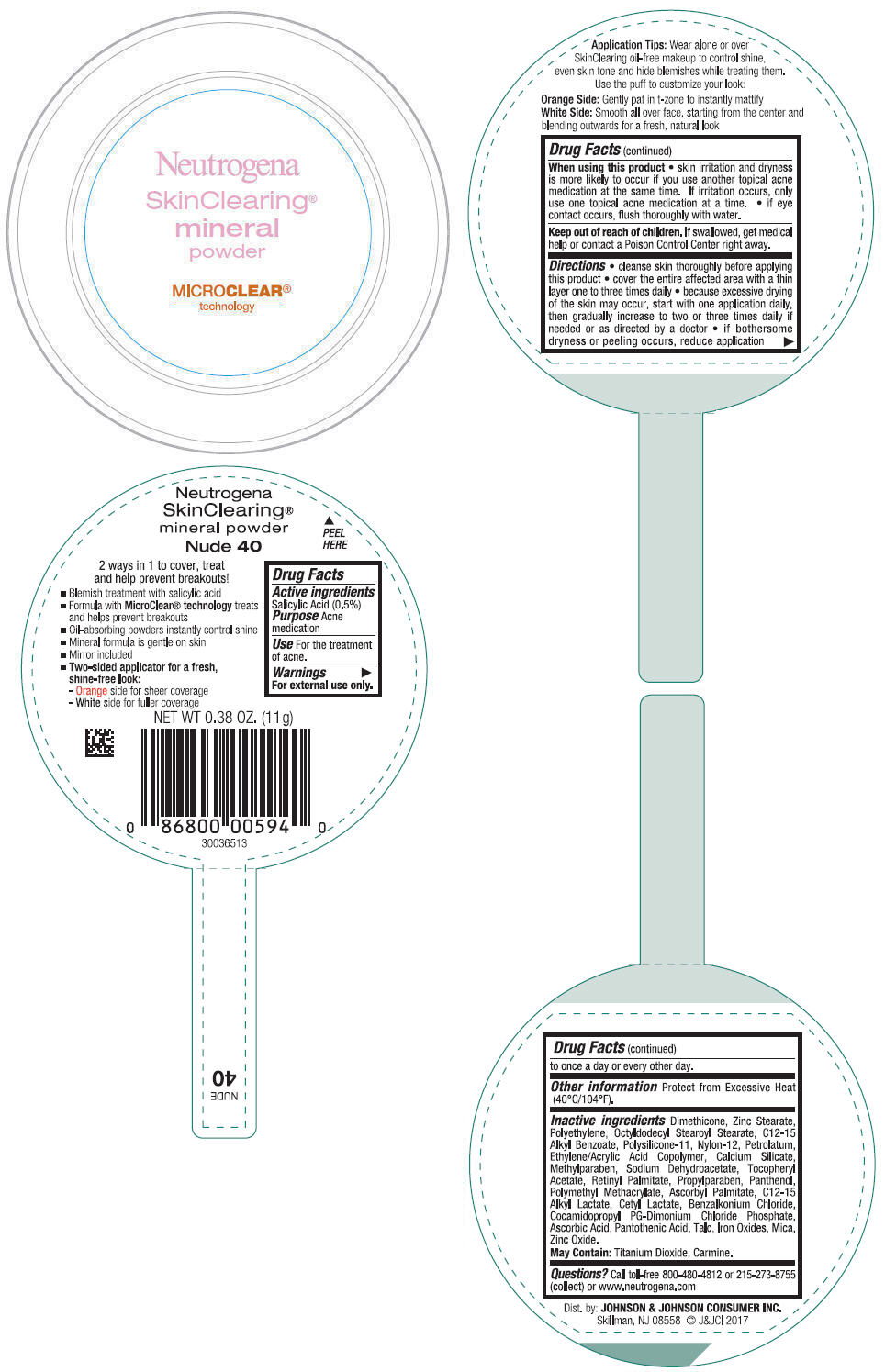
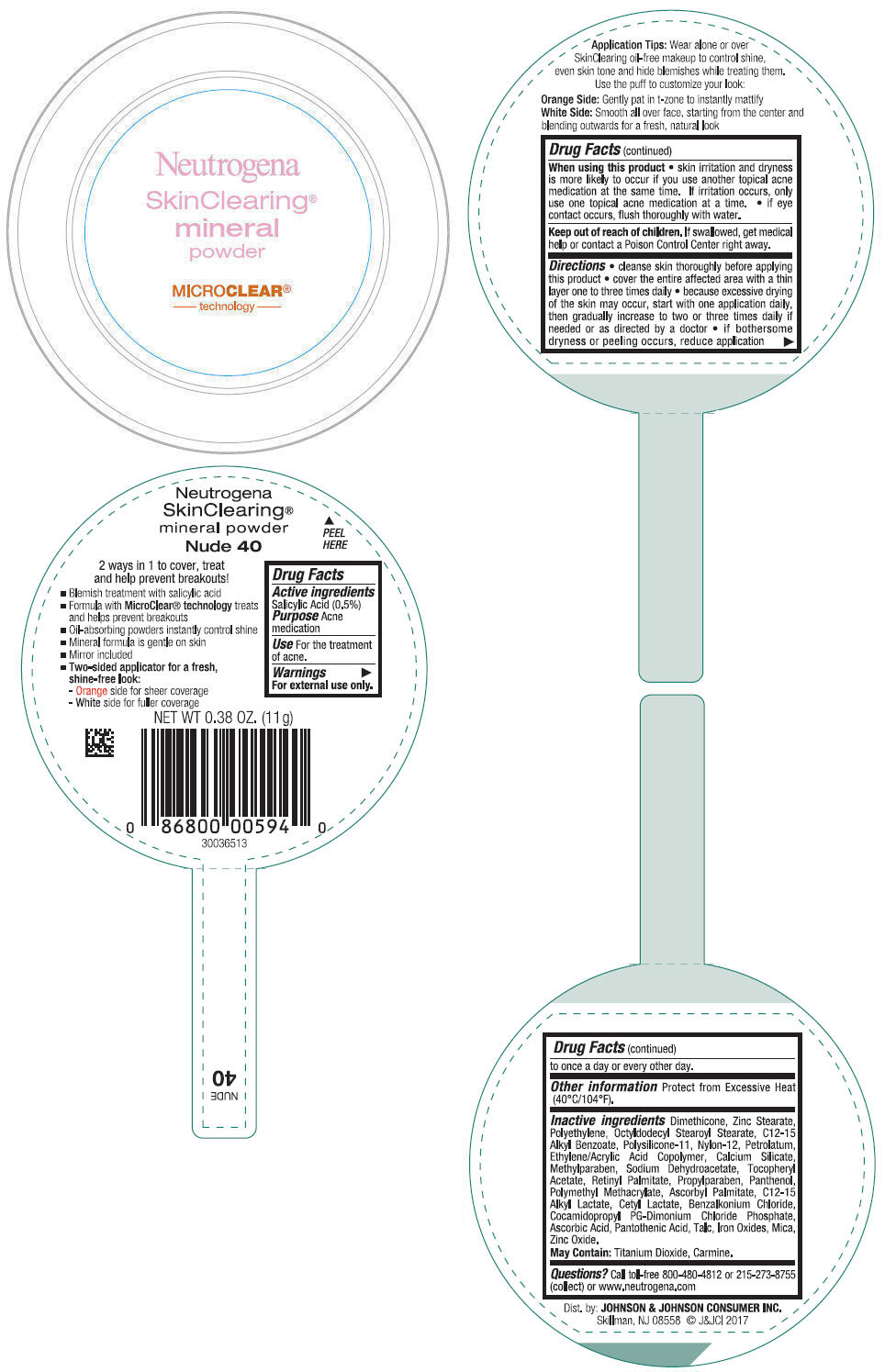
NEUTROGENA SKINCLEARING MINERAL - NUDE- salicylic acid powder
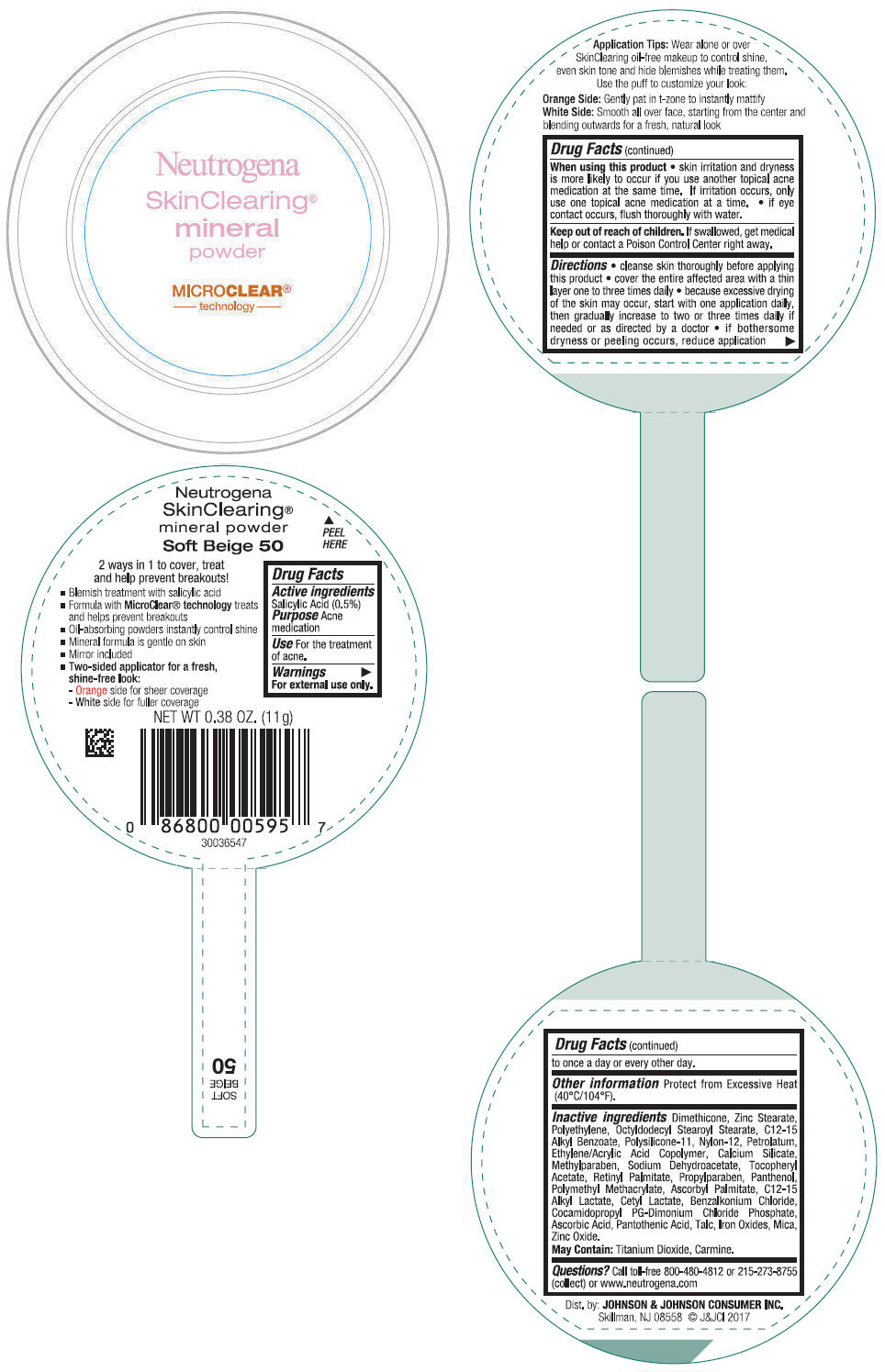
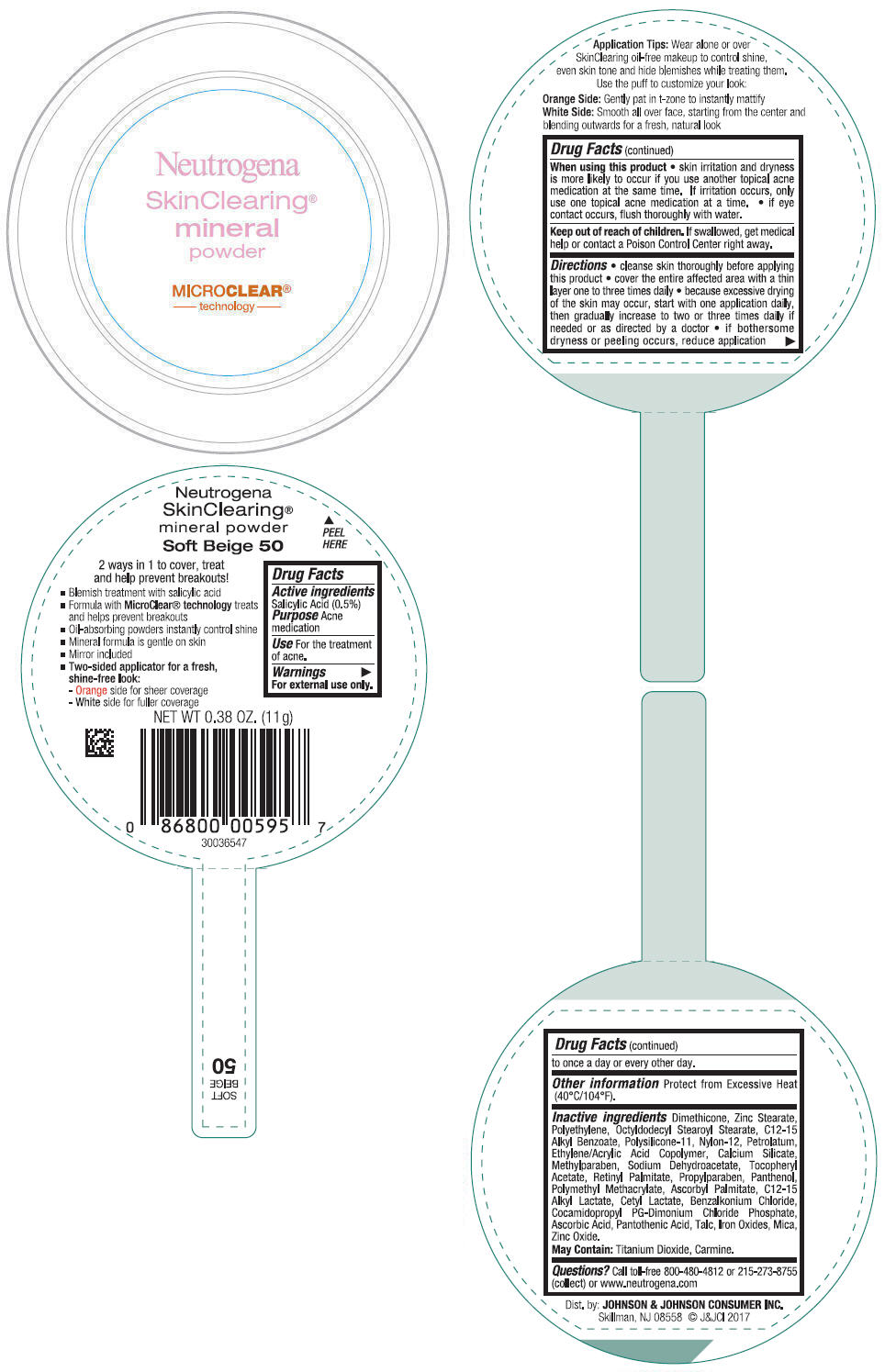
NEUTROGENA SKINCLEARING MINERAL - SOFT BEIGE- salicylic acid powder
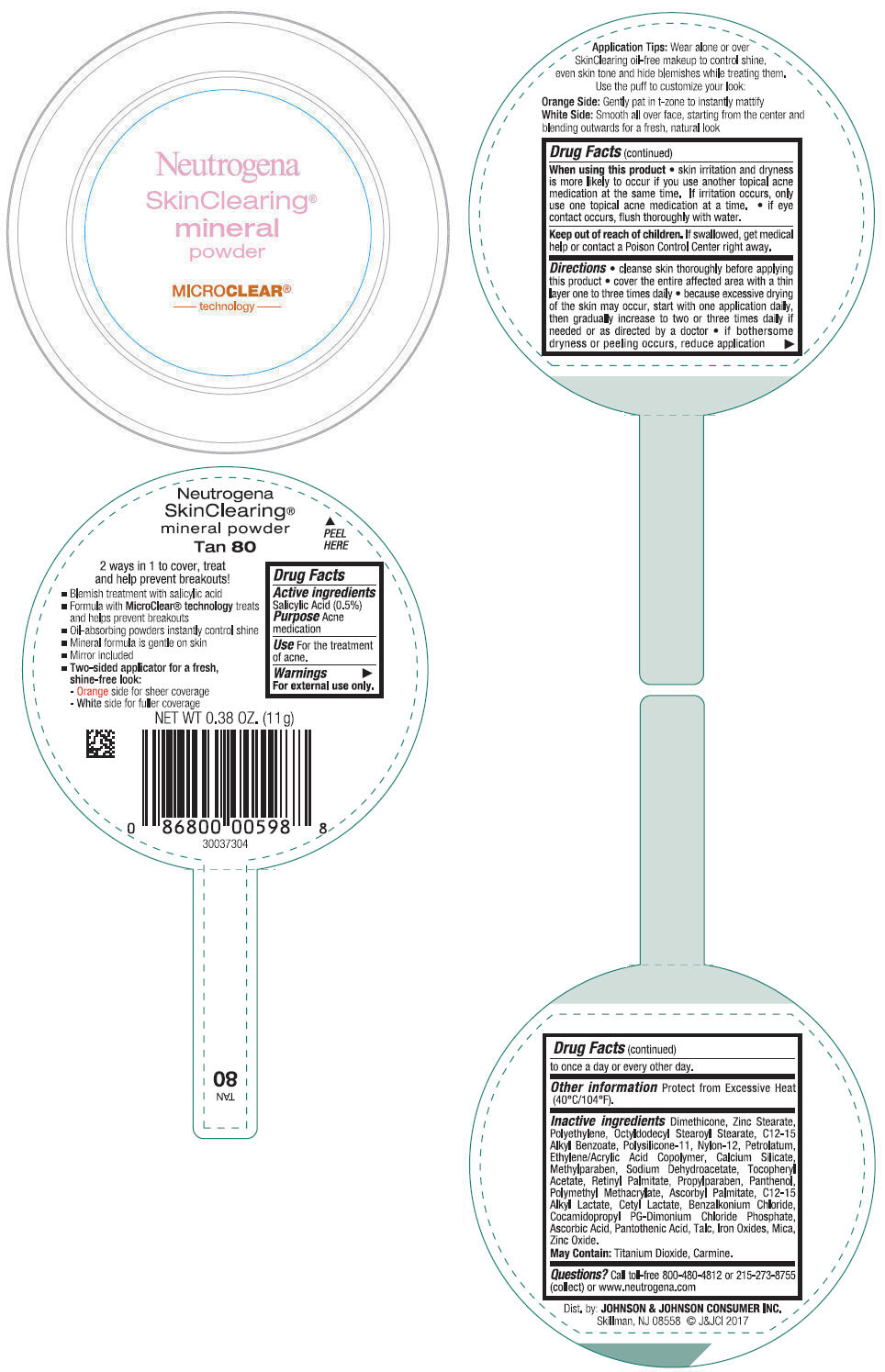
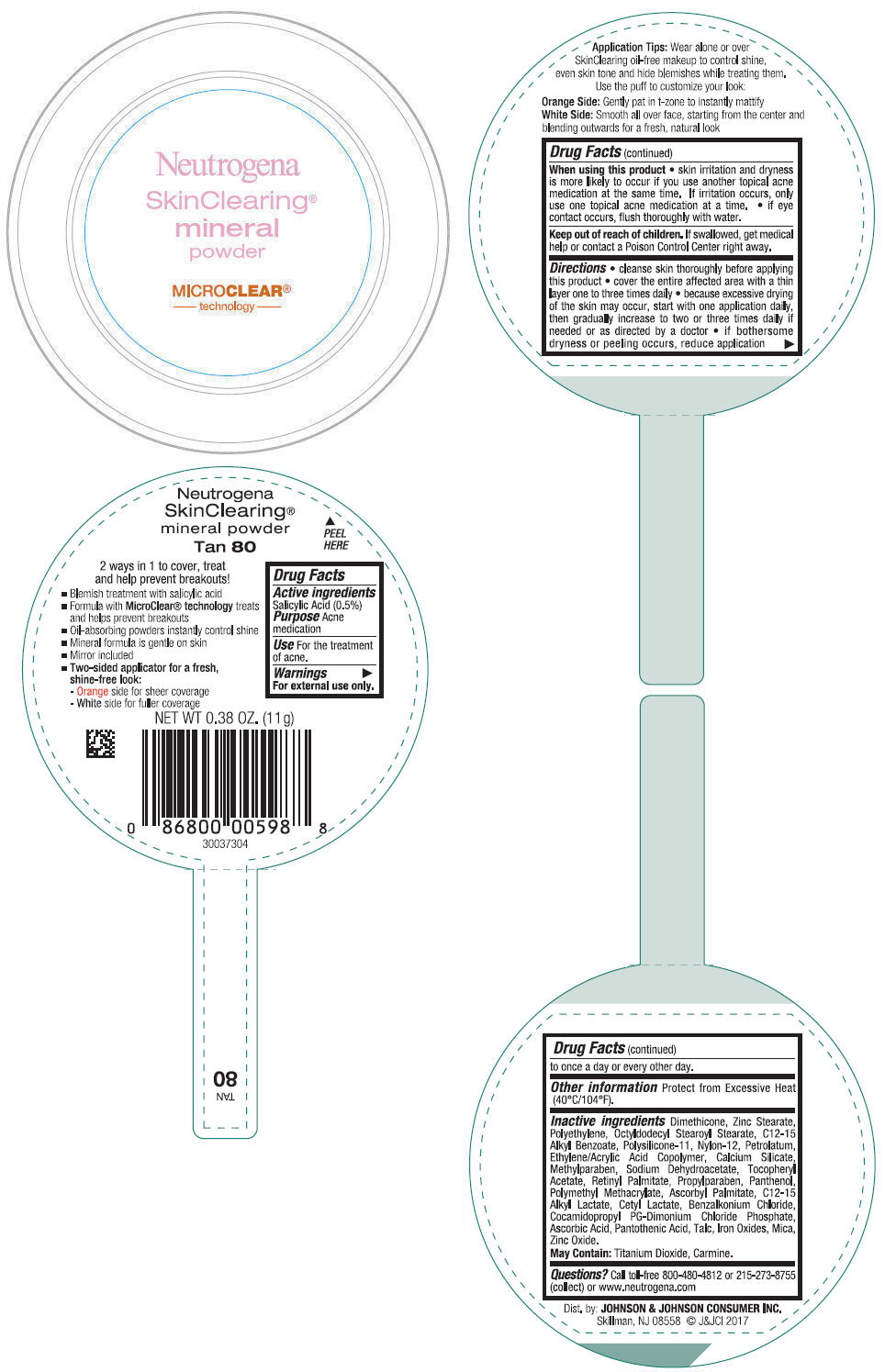
NEUTROGENA SKINCLEARING MINERAL - TAN- salicylic acid powder
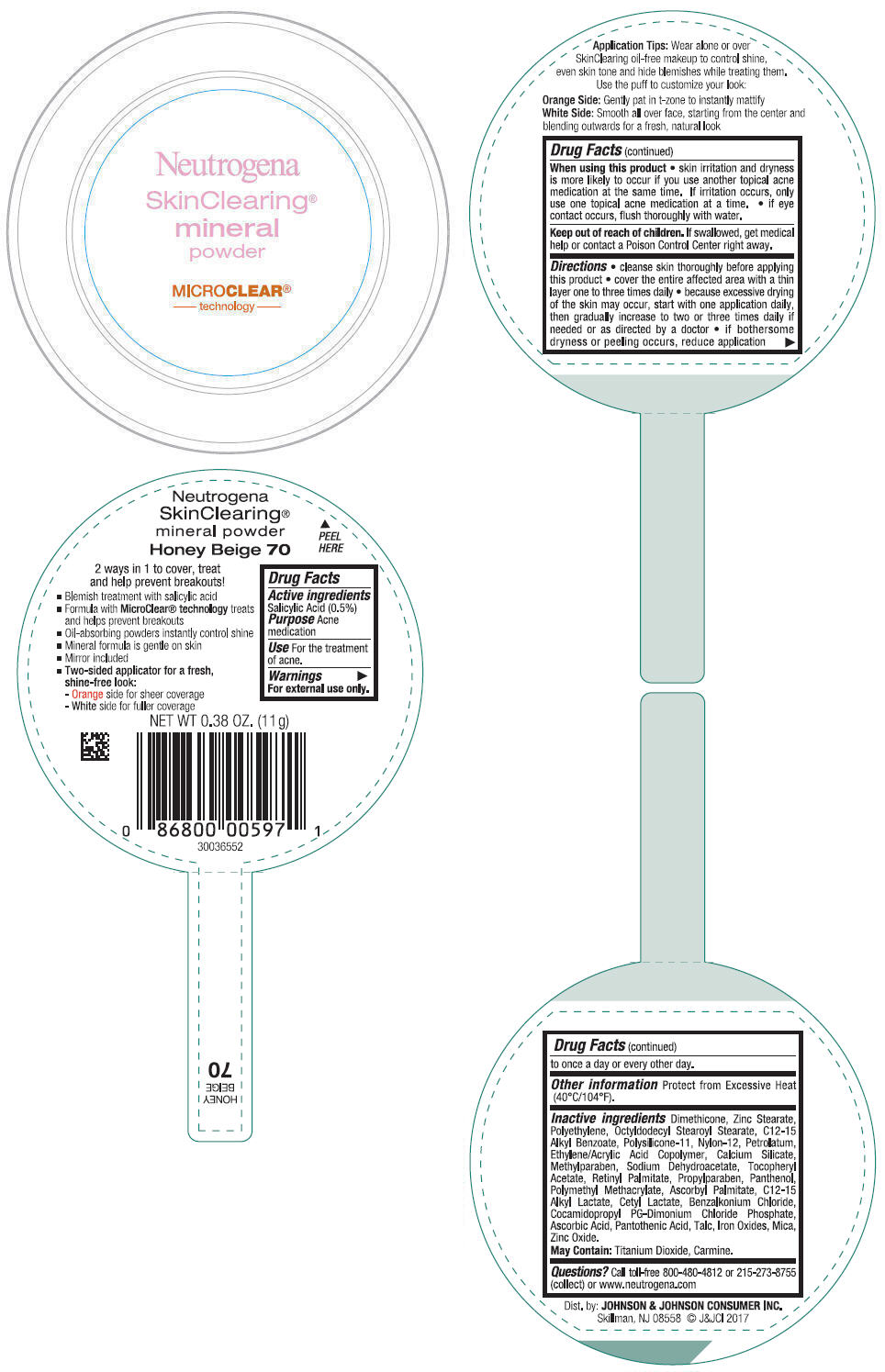
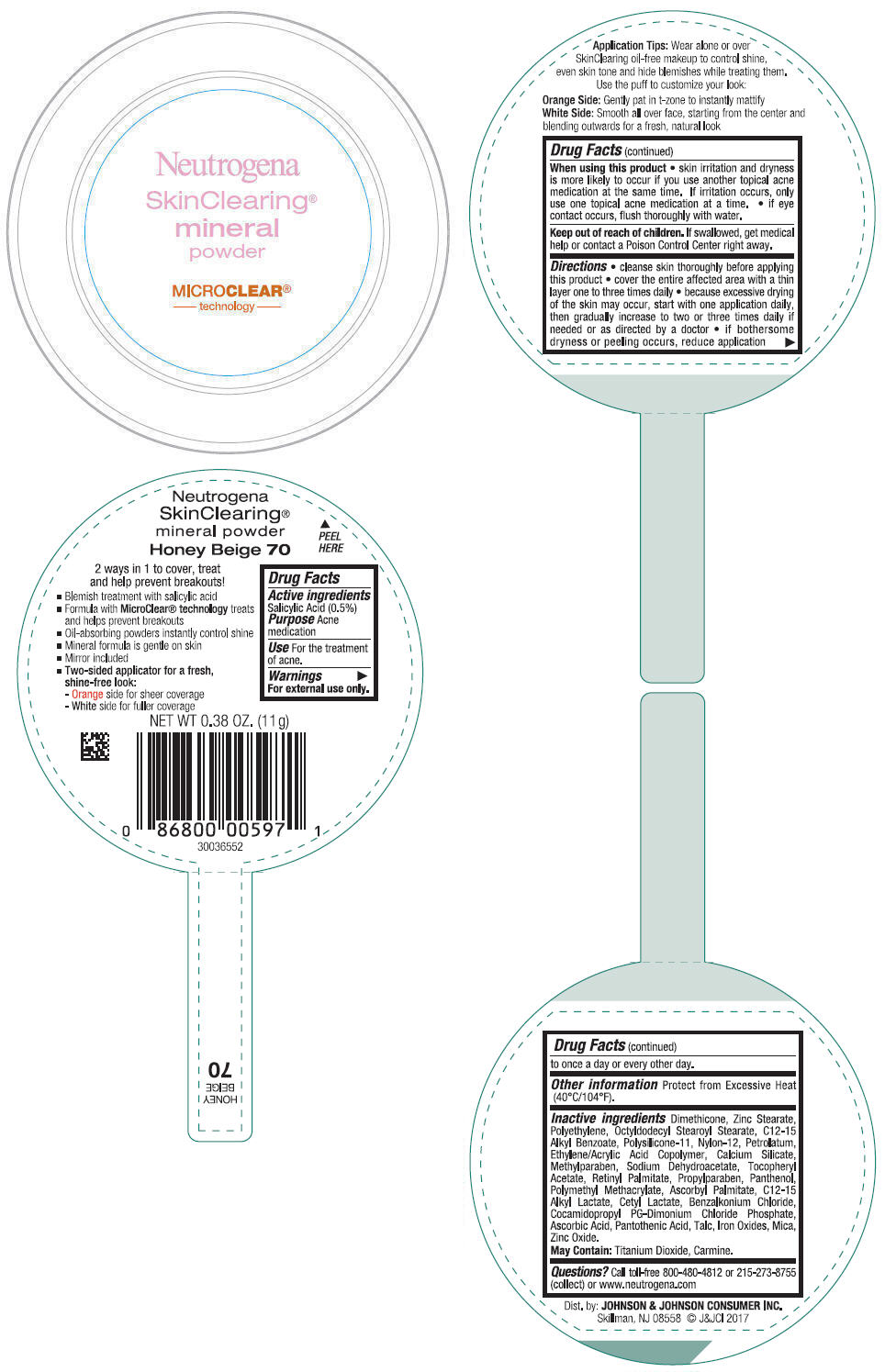
NEUTROGENA SKINCLEARING MINERAL - HONEY BEIGE- salicylic acid powder
-
NDC Code(s):
69968-0264-1,
69968-0265-1,
69968-0266-1,
69968-0267-1, view more69968-0268-1, 69968-0309-1, 69968-0310-1, 69968-0311-1
- Packager: Kenvue Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Use
- Warnings
-
Directions
- cleanse skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- Other information
-
Inactive ingredients
Dimethicone, Zinc Stearate, Polyethylene, Octyldodecyl Stearoyl Stearate, C12-15 Alkyl Benzoate, Polysilicone-11, Nylon-12, Petrolatum, Ethylene/Acrylic Acid Copolymer, Calcium Silicate, Methylparaben, Sodium Dehydroacetate, Tocopheryl Acetate, Retinyl Palmitate, Propylparaben, Panthenol, Polymethyl Methacrylate, Ascorbyl Palmitate, C12-15 Alkyl Lactate, Cetyl Lactate, Benzalkonium Chloride, Cocamidopropyl PG-Dimonium Chloride Phosphate, Ascorbic Acid, Pantothenic Acid, Talc, Iron Oxides, Mica, Zinc Oxide.
May Contain: Titanium Dioxide, Carmine.
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 11 g Container Label - Buff 30
- PRINCIPAL DISPLAY PANEL - 11 g Container Label - Classic Ivory 10
- PRINCIPAL DISPLAY PANEL - 11 g Container Label - Natural Beige 60
- PRINCIPAL DISPLAY PANEL - 11 g Container Label - Natural Ivory 20
- PRINCIPAL DISPLAY PANEL - 11 g Container Label - Nude 40
- PRINCIPAL DISPLAY PANEL - 11 g Container Label - Soft Beige 50
- PRINCIPAL DISPLAY PANEL - 11 g Container Label - Tan 80
- PRINCIPAL DISPLAY PANEL - 11 g Container Label - Honey Beige 70
-
INGREDIENTS AND APPEARANCE
NEUTROGENA SKINCLEARING MINERAL - BUFF
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0267 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) DIMETHICONE (UNII: 92RU3N3Y1O) ZINC STEARATE (UNII: H92E6QA4FV) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) MICA (UNII: V8A1AW0880) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) NYLON-12 (UNII: 446U8J075B) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PETROLATUM (UNII: 4T6H12BN9U) ACRYLIC ACID/ETHYLENE COPOLYMER (600 MPA.S) (UNII: 1PEZ3NLY6I) CALCIUM SILICATE (UNII: S4255P4G5M) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) METHYLPARABEN (UNII: A2I8C7HI9T) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLPARABEN (UNII: Z8IX2SC1OH) PANTHENOL (UNII: WV9CM0O67Z) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) COCAMIDOPROPYL PROPYLENE GLYCOL-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) CETYL LACTATE (UNII: A7EVH2RK4O) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) ASCORBYL PALMITATE (UNII: QN83US2B0N) PANTOTHENIC ACID (UNII: 19F5HK2737) ASCORBIC ACID (UNII: PQ6CK8PD0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0267-1 11 g in 1 CONTAINER; Type 0: Not a Combination Product 11/30/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 11/30/2009 NEUTROGENA SKINCLEARING MINERAL - CLASSIC IVORY
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0309 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) DIMETHICONE (UNII: 92RU3N3Y1O) ZINC STEARATE (UNII: H92E6QA4FV) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) MICA (UNII: V8A1AW0880) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) NYLON-12 (UNII: 446U8J075B) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PETROLATUM (UNII: 4T6H12BN9U) ACRYLIC ACID/ETHYLENE COPOLYMER (600 MPA.S) (UNII: 1PEZ3NLY6I) CALCIUM SILICATE (UNII: S4255P4G5M) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) METHYLPARABEN (UNII: A2I8C7HI9T) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLPARABEN (UNII: Z8IX2SC1OH) PANTHENOL (UNII: WV9CM0O67Z) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) COCAMIDOPROPYL PROPYLENE GLYCOL-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) CETYL LACTATE (UNII: A7EVH2RK4O) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) ASCORBYL PALMITATE (UNII: QN83US2B0N) PANTOTHENIC ACID (UNII: 19F5HK2737) ASCORBIC ACID (UNII: PQ6CK8PD0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0309-1 11 g in 1 CONTAINER; Type 0: Not a Combination Product 11/30/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 11/30/2009 NEUTROGENA SKINCLEARING MINERAL - NATURAL BEIGE
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0310 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) DIMETHICONE (UNII: 92RU3N3Y1O) ZINC STEARATE (UNII: H92E6QA4FV) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) MICA (UNII: V8A1AW0880) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) NYLON-12 (UNII: 446U8J075B) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PETROLATUM (UNII: 4T6H12BN9U) ACRYLIC ACID/ETHYLENE COPOLYMER (600 MPA.S) (UNII: 1PEZ3NLY6I) CALCIUM SILICATE (UNII: S4255P4G5M) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) METHYLPARABEN (UNII: A2I8C7HI9T) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLPARABEN (UNII: Z8IX2SC1OH) PANTHENOL (UNII: WV9CM0O67Z) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) COCAMIDOPROPYL PROPYLENE GLYCOL-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) CETYL LACTATE (UNII: A7EVH2RK4O) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) ASCORBYL PALMITATE (UNII: QN83US2B0N) PANTOTHENIC ACID (UNII: 19F5HK2737) ASCORBIC ACID (UNII: PQ6CK8PD0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0310-1 11 g in 1 CONTAINER; Type 0: Not a Combination Product 11/30/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 11/30/2009 NEUTROGENA SKINCLEARING MINERAL - NATURAL IVORY
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0268 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) DIMETHICONE (UNII: 92RU3N3Y1O) ZINC STEARATE (UNII: H92E6QA4FV) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) MICA (UNII: V8A1AW0880) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) NYLON-12 (UNII: 446U8J075B) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PETROLATUM (UNII: 4T6H12BN9U) ACRYLIC ACID/ETHYLENE COPOLYMER (600 MPA.S) (UNII: 1PEZ3NLY6I) CALCIUM SILICATE (UNII: S4255P4G5M) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) METHYLPARABEN (UNII: A2I8C7HI9T) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLPARABEN (UNII: Z8IX2SC1OH) PANTHENOL (UNII: WV9CM0O67Z) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) COCAMIDOPROPYL PROPYLENE GLYCOL-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) CETYL LACTATE (UNII: A7EVH2RK4O) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) ASCORBYL PALMITATE (UNII: QN83US2B0N) PANTOTHENIC ACID (UNII: 19F5HK2737) ASCORBIC ACID (UNII: PQ6CK8PD0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0268-1 11 g in 1 CONTAINER; Type 0: Not a Combination Product 11/30/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 11/30/2009 NEUTROGENA SKINCLEARING MINERAL - NUDE
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0266 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) DIMETHICONE (UNII: 92RU3N3Y1O) ZINC STEARATE (UNII: H92E6QA4FV) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) MICA (UNII: V8A1AW0880) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) NYLON-12 (UNII: 446U8J075B) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PETROLATUM (UNII: 4T6H12BN9U) ACRYLIC ACID/ETHYLENE COPOLYMER (600 MPA.S) (UNII: 1PEZ3NLY6I) CALCIUM SILICATE (UNII: S4255P4G5M) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) METHYLPARABEN (UNII: A2I8C7HI9T) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLPARABEN (UNII: Z8IX2SC1OH) PANTHENOL (UNII: WV9CM0O67Z) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) COCAMIDOPROPYL PROPYLENE GLYCOL-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) CETYL LACTATE (UNII: A7EVH2RK4O) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) ASCORBYL PALMITATE (UNII: QN83US2B0N) PANTOTHENIC ACID (UNII: 19F5HK2737) ASCORBIC ACID (UNII: PQ6CK8PD0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0266-1 11 g in 1 CONTAINER; Type 0: Not a Combination Product 11/30/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 11/30/2009 NEUTROGENA SKINCLEARING MINERAL - SOFT BEIGE
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0265 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) DIMETHICONE (UNII: 92RU3N3Y1O) ZINC STEARATE (UNII: H92E6QA4FV) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) MICA (UNII: V8A1AW0880) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) NYLON-12 (UNII: 446U8J075B) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PETROLATUM (UNII: 4T6H12BN9U) ACRYLIC ACID/ETHYLENE COPOLYMER (600 MPA.S) (UNII: 1PEZ3NLY6I) CALCIUM SILICATE (UNII: S4255P4G5M) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) METHYLPARABEN (UNII: A2I8C7HI9T) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLPARABEN (UNII: Z8IX2SC1OH) PANTHENOL (UNII: WV9CM0O67Z) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) COCAMIDOPROPYL PROPYLENE GLYCOL-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) CETYL LACTATE (UNII: A7EVH2RK4O) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) ASCORBYL PALMITATE (UNII: QN83US2B0N) PANTOTHENIC ACID (UNII: 19F5HK2737) ASCORBIC ACID (UNII: PQ6CK8PD0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0265-1 11 g in 1 CONTAINER; Type 0: Not a Combination Product 11/30/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 11/30/2009 NEUTROGENA SKINCLEARING MINERAL - TAN
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0311 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) DIMETHICONE (UNII: 92RU3N3Y1O) ZINC STEARATE (UNII: H92E6QA4FV) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) MICA (UNII: V8A1AW0880) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) NYLON-12 (UNII: 446U8J075B) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PETROLATUM (UNII: 4T6H12BN9U) ACRYLIC ACID/ETHYLENE COPOLYMER (600 MPA.S) (UNII: 1PEZ3NLY6I) CALCIUM SILICATE (UNII: S4255P4G5M) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) METHYLPARABEN (UNII: A2I8C7HI9T) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLPARABEN (UNII: Z8IX2SC1OH) PANTHENOL (UNII: WV9CM0O67Z) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) COCAMIDOPROPYL PROPYLENE GLYCOL-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) CETYL LACTATE (UNII: A7EVH2RK4O) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) ASCORBYL PALMITATE (UNII: QN83US2B0N) PANTOTHENIC ACID (UNII: 19F5HK2737) ASCORBIC ACID (UNII: PQ6CK8PD0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0311-1 11 g in 1 CONTAINER; Type 0: Not a Combination Product 11/30/2009 01/31/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 11/30/2009 01/31/2020 NEUTROGENA SKINCLEARING MINERAL - HONEY BEIGE
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0264 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength PANTHENOL (UNII: WV9CM0O67Z) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) COCAMIDOPROPYL PROPYLENE GLYCOL-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) CETYL LACTATE (UNII: A7EVH2RK4O) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) ASCORBYL PALMITATE (UNII: QN83US2B0N) PANTOTHENIC ACID (UNII: 19F5HK2737) ASCORBIC ACID (UNII: PQ6CK8PD0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) ZINC OXIDE (UNII: SOI2LOH54Z) TALC (UNII: 7SEV7J4R1U) DIMETHICONE (UNII: 92RU3N3Y1O) ZINC STEARATE (UNII: H92E6QA4FV) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) MICA (UNII: V8A1AW0880) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) NYLON-12 (UNII: 446U8J075B) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PETROLATUM (UNII: 4T6H12BN9U) ACRYLIC ACID/ETHYLENE COPOLYMER (600 MPA.S) (UNII: 1PEZ3NLY6I) CALCIUM SILICATE (UNII: S4255P4G5M) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) METHYLPARABEN (UNII: A2I8C7HI9T) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0264-1 11 g in 1 CONTAINER; Type 0: Not a Combination Product 11/30/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 11/30/2009 Labeler - Kenvue Brands LLC (118772437)