Label: PERTZYE- pancrelipase capsule, delayed release
-
NDC Code(s):
59767-004-01,
59767-004-99,
59767-008-00,
59767-008-01, view more59767-008-02, 59767-008-99, 59767-016-00, 59767-016-01, 59767-016-02, 59767-016-99, 59767-024-00, 59767-024-01, 59767-024-02, 59767-024-99
- Packager: Digestive Care, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated March 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PERTZYE safely and effectively. See full prescribing information for PERTZYE.
PERTZYE (pancrelipase) delayed-release capsules, for oral use
Initial U.S. Approval: 2012INDICATIONS AND USAGE
PERTZYE® is indicated for the treatment of exocrine pancreatic insufficiency in adult and pediatric patients. (1)
DOSAGE AND ADMINISTRATION
Important Dosing Information (2.1)
- PERTZYE is a mixture of enzymes including lipases, proteases, and amylases and dosing is based on lipase units. Dosing scheme based on actual body weight or fat ingestion.
- Individualize the dosage based on clinical symptoms, the degree of steatorrhea present, and the fat content of the diet.
- Do not exceed 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or 4,000 lipase units/g fat ingested/day in adult and pediatric patients greater than 12 months of age without further investigation. (5.1)
- The total daily dosage in adult and pediatric patients greater than 12 months of age should reflect approximately three meals plus two or three snacks per day. With each snack, administer approximately half the prescribed dose for a meal.
- Do not substitute other pancreatic enzyme products for PERTZYE. When switching from another pancreatic enzyme product to PERTZYE, monitor patients for clinical symptoms of exocrine pancreatic insufficiency and titrate the dosage as needed.
Recommended Dosage (2.2)
Adults and Pediatric Patients Greater than 12 Months: The recommended initial starting dosage is:
- 500 lipase units/kg/meal for adult and pediatric patients 4 years of age and older.
- 1,000 lipase units/kg/meal for pediatric patients greater than 12 months to less than 4 years of age.
- Titrate the dosage to either 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or 4,000 lipase units/g fat ingested/day. Higher dosages may be administered if documented to be effective by fecal fat measures or improvement in malabsorption.
Pediatric Patients Birth to 12 Months: The recommended dosage is 4,000 lipase units (one capsule) per 120 mL of formula or per breastfeeding.
Preparation and Administration Instructions (2.3)
- Swallow capsules whole. For patients unable to swallow intact capsule(s), the capsule contents may be sprinkled on soft acidic food (e.g., applesauce).
- The 4,000 USP lipase unit capsule may be administered with applesauce via gastrostomy tube (14 French or larger). The contents of no more than two capsules may be administered at a time.
- Do not crush or chew PERTZYE capsules or capsule contents.
- Consume sufficient liquids to ensure complete swallowing of PERTZYE. (5.2)
- See the full prescribing information for additional information on administering to pediatric patients birth to 12 months of age.
DOSAGE FORMS AND STRENGTHS
Delayed-Release Capsules (3):
- 4,000 USP units of lipase; 14,375 USP units of protease; and 15,125 USP units of amylase.
- 8,000 USP units of lipase; 28,750 USP units of protease; and 30,250 USP units of amylase.
- 16,000 USP units of lipase; 57,500 USP units of protease; and 60,500 USP units of amylase.
- 24,000 USP units of lipase; 86,250 USP units of protease; and 90,750 USP units of amylase.
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Fibrosing Colonopathy: Associated with high doses, usually over prolonged use and in pediatric patients with cystic fibrosis. Colonic stricture reported in pediatric patients less than 12 years of age with dosages exceeding 6,000 lipase units/kg/meal. Monitor during treatment for progression of preexisting disease. Do not exceed the recommended dosage, unless clinically indicated. (2.1, 5.1)
- Irritation of the Oral Mucosa: May occur due to loss of protective enteric coating on the capsule contents. (5.2)
- Hyperuricemia: Reported with high dosages, consider monitoring blood uric acid levels in patients with gout, renal impairment, or hyperuricemia. (5.3)
- Risk of Viral Transmission: The presence of porcine viruses that might infect humans cannot be definitely excluded. (5.4)
- Hypersensitivity Reactions: Monitor patients with known reactions to proteins of porcine origin. If symptoms occur, initiate appropriate medical management; consider the risks and benefits of continued treatment. (5.5)
ADVERSE REACTIONS
Most common adverse reactions (≥ 10%) are: diarrhea, dyspepsia, and cough. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Digestive Care Inc. at 1-877-882-5950 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Information
2.2 Recommended Dosage
2.3 Preparation and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Fibrosing Colonopathy
5.2 Irritation of the Oral Mucosa
5.3 Hyperuricemia
5.4 Risk of Viral Transmission
5.5 Hypersensitivity Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Information
PERTZYE is a mixture of enzymes including lipases, proteases, and amylases. PERTZYE dosing is based on lipase units.
- Use either an actual body weight or fat ingestion-based dosing scheme.
- Start at the lowest recommended dosage and individualize the dosage based on clinical symptoms, the degree of steatorrhea present, and the fat content of the diet. Changes in dosage may require an adjustment period of several days.
- Do not exceed 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or 4,000 lipase units/g fat ingested/day in adult and pediatric patients greater than 12 months of age without further investigation [see Warnings and Precautions (5.1)].
- The total daily dosage in adult and pediatric patients greater than 12 months of age should reflect approximately three meals plus two or three snacks per day. With each snack, administer approximately half the prescribed PERTZYE dose for a meal.
- Do not substitute other pancreatic enzyme products for PERTZYE. When switching from another pancreatic enzyme product to PERTZYE, monitor patients for clinical symptoms of exocrine pancreatic insufficiency and titrate the dosage as needed.
2.2 Recommended Dosage
Adults and Pediatric Patients Greater than 12 Months of Age
The recommended oral initial starting dosage is:
- 500 lipase units/kg/meal for adult and pediatric patients 4 years of age and older.
- 1,000 lipase units/kg/meal for pediatric patients greater than 12 months to less than 4 years of age.
If signs and symptoms of malabsorption persist, increase the dosage. Titrate to either 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or 4,000 lipase units/gram of fat ingested/day. Higher dosages may be administered if they are documented to be effective by fecal fat measures or an improvement in signs or symptoms of malabsorption including measures of nutritional status.
2.3 Preparation and Administration Instructions
Adult and Pediatric Patients Greater than 12 Months of Age
Instruct adult and pediatric patients greater than 12 months of age, or their caregivers, of the following:
- Take PERTZYE with meals or snacks. If a dose is missed, take the next dose with the next meal or snack.
- Swallow capsules whole.
- For patients unable to swallow intact capsules, the capsule contents may be sprinkled on a small amount of soft acidic food with a pH of 4.5 or less (e.g., applesauce). The 4,000 USP lipase unit capsule may also be administered with applesauce via gastrostomy tube 14 French or larger.
- Do not crush or chew PERTZYE capsules or capsule contents.
- Consume sufficient liquids (water or juice) to ensure complete swallowing of PERTZYE [see Warnings and Precautions (5.2)].
Oral Administration for Patients Unable to Swallow Intact Capsules
- Carefully open the capsules and sprinkle the entire contents on a small amount of acidic soft food (approximately 10 mL) with a pH of 4.5 or less (e.g., applesauce).
- Mix the capsule contents with the acidic soft food (e.g., applesauce) being careful not to crush the contents.
- Consume the entire mixture immediately. Do not save the mixture for later use.
- Consume sufficient fluids to ensure complete swallowing of the PERTZYE food mixture.
Gastrostomy Tube Administration (14 French Gastrostomy Tube or Larger)
- Only perform gastrostomy tube administration with the contents of the 4,000 USP lipase unit capsule of PERTZYE.
- The contents of no more than two capsules may be administered at a time in the gastrostomy tube.
- Transfer a minimum of 10 mL of applesauce into a small bowl or medicine cup.
- Carefully open one or two PERTZYE 4,000 lipase unit capsules.
- Mix the capsule contents thoroughly with the transferred applesauce to create a uniform suspension being careful not to crush the contents. Once mixed, administer the suspension immediately.
- Remove the plunger from a 35 mL slip tip syringe. Cover the tip of the syringe with your finger. Transfer the PERTZYE-applesauce mixture into the syringe. Replace the plunger partially back into the syringe.
- Shake or tap the syringe lightly with the syringe tip facing upward so that the PERTZYE-applesauce mixture will move towards the plunger. Carefully push the plunger slowly until the residual air is removed from the syringe tip.
- Once the residual air is removed, connect the syringe directly into the gastrostomy tube feeding port.
- Push the syringe contents into the gastrostomy tube feeding port using steady pressure until empty.
- Draw up approximately 10 mL of water with the slip tip syringe and flush the gastrostomy tube feeding port with the water.
- Discard any unused portion of the PERTZYE-applesauce mixture. Do not save for later use.
- If dose requires more than two capsules, repeat steps 1-9 until prescribed dose is reached.
Pediatric Patients Birth to 12 Months of Age:
Instruct caregivers of pediatric patients birth to 12 months of age of the following:
- Immediately prior to each breastfeeding session or each administration of 120 mL of formula, carefully open one PERTZYE capsule (containing 4,000 USP units of lipase) and administer the entire contents using one of the following two methods:
- Sprinkle on a small amount of acidic soft food with a pH of 4.5 or less (e.g., applesauce) being careful not to crush the capsule contents. The entire mixture should be given to the infant immediately.
- Sprinkle the capsule contents directly into the infant's mouth.
- Immediately administer breast milk or formula after PERTZYE to ensure complete swallowing of the PERTZYE capsule contents.
- Do not crush PERTZYE capsule contents, and visually inspect the infant's mouth to ensure that no drug is retained in the mouth [see Warnings and Precautions (5.2)].
- If a dose is missed, administer the next dose with the next feeding.
-
3 DOSAGE FORMS AND STRENGTHS
Delayed-release capsules are available in the following strengths:
- 4,000 USP units of lipase; 14,375 USP units of protease; and 15,125 USP units of amylase in a two-piece hard gelatin capsule with a clear body printed in green with "4" and a clear cap printed with a green circular stripe and "DCI"
- 8,000 USP units of lipase; 28,750 USP units of protease; and 30,250 USP units of amylase in a two-piece hard gelatin capsule with a clear body printed in blue with "8" and a clear cap printed with a blue circular stripe and "DCI"
- 16,000 USP units of lipase; 57,500 USP units of protease; and 60,500 USP units of amylase in a two-piece hard gelatin capsule with a clear body printed in red with "16" and a clear cap printed with a red circular stripe and "DCI"
- 24,000 USP units of lipase; 86,250 USP units of protease; and 90,750 USP units of amylase in a two-piece hard gelatin capsule with a clear body printed in purple with "24" and a clear cap printed with a purple circular stripe and "DCI"
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Fibrosing Colonopathy
Fibrosing colonopathy has been reported following treatment with pancreatic enzyme products. Fibrosing colonopathy is a rare, serious adverse reaction initially described in association with use of high-dose pancreatic enzyme products, usually with use over a prolonged period of time and most commonly reported in pediatric patients with cystic fibrosis. Pancreatic enzyme products exceeding 6,000 lipase units/kg/meal have been associated with colonic stricture, a complication of fibrosing colonopathy, in pediatric patients less than 12 years of age. The underlying mechanism of fibrosing colonopathy remains unknown.
If there is a history of fibrosing colonopathy, monitor patients during treatment with PERTZYE because some patients may be at risk of progressing to colonic stricture formation. It is uncertain whether regression of fibrosing colonopathy occurs. Do not exceed the recommended dosage of either 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or 4,000 lipase units/g fat ingested/day in adult and pediatric patients greater than 12 months of age without further investigation. Higher dosages may be administered if they are documented to be effective by fecal fat measures or an improvement in signs and symptoms of malabsorption including measures of nutritional status. Patients receiving dosages higher than 6,000 lipase units/kg/meal should be frequently monitored for symptoms of fibrosing colonopathy and the dosage decreased or titrated downward to a lower range if clinically appropriate [see Dosage and Administration (2.1)].
5.2 Irritation of the Oral Mucosa
Crushing or chewing PERTZYE capsules or mixing the capsule contents in foods having a pH greater than 4.5 can disrupt the protective enteric coating on the capsule contents and result in early release of enzymes, irritation of the oral mucosa, and/or loss of enzyme activity.
Instruct the patient or caregiver of the following:
- Swallow capsules whole. For patients who cannot swallow the capsules whole, the capsules can be opened, and the contents sprinkled on a small amount of acidic soft food with a pH of 4.5 or less (e.g., applesauce). The 4,000 USP lipase unit capsule may also be administered with applesauce via a gastrostomy tube with a diameter of 14 French or larger.
- Do not crush or chew PERTZYE capsules or capsule contents.
- Consume sufficient liquids (juice, water, breast milk, or formula) immediately following administration of PERTZYE to ensure complete swallowing.
- Visually inspect the mouth of pediatric patients less than 12 months of age and of patients who are unable to swallow intact capsules to ensure no drug is retained in the mouth and irritation of the oral mucosa has not occurred [see Dosage and Administration (2.3)].
5.3 Hyperuricemia
Pancreatic enzyme products contain purines that may increase blood uric acid levels. High dosages have been associated with hyperuricosuria and hyperuricemia [see Overdosage (10)]. Consider monitoring blood uric acid levels in patients with gout, renal impairment, or hyperuricemia during treatment with PERTZYE.
5.4 Risk of Viral Transmission
PERTZYE is sourced from pancreatic tissue from swine used for food consumption. Although the risk that PERTZYE will transmit an infectious agent to humans has been reduced by testing for certain viruses during manufacturing and by inactivating certain viruses during manufacturing, there is a theoretical risk for transmission of viral disease, including diseases caused by novel or unidentified viruses. Thus, the presence of porcine viruses that might infect humans cannot be definitely excluded. However, no cases of transmission of an infectious illness associated with the use of porcine pancreatic extracts have been reported.
5.5 Hypersensitivity Reactions
Severe hypersensitivity reactions including anaphylaxis, asthma, hives, and pruritus have been reported with pancreatic enzyme products [see Adverse Reactions (6.2)]. If symptoms occur, initiate appropriate medical management.
Monitor patients with a known hypersensitivity reaction to proteins of porcine origin for hypersensitivity reactions during treatment with PERTZYE. The risks and benefits of continued PERTZYE treatment in patients with severe hypersensitivity reactions should be taken into consideration with the overall clinical needs of the patient.
-
6 ADVERSE REACTIONS
The following serious or otherwise important adverse reactions are described elsewhere in the labeling:
- Fibrosing Colonopathy [see Warnings and Precautions (5.1)]
- Irritation of the Oral Mucosa [see Warnings and Precautions (5.2)]
- Hyperuricemia [see Warnings and Precautions (5.3)]
- Risk of Viral Transmission [see Warnings and Precautions (5.4)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data described below reflect exposure to PERTZYE in 21 patients, aged 8 to 43 years, with exocrine pancreatic insufficiency due to cystic fibrosis in a placebo-controlled clinical trial [see Clinical Studies (14)].
Table 1 enumerates adverse reactions that occurred in at least 2 patients (greater than or equal to 10%) treated with PERTZYE at a higher rate than with placebo.
Table 1. Adverse Reactions* in a Clinical Trial of Adult and Pediatric Patients 8 Years of Age and Older with Exocrine Pancreatic Insufficiency Due to Cystic Fibrosis Adverse Reaction PERTZYE
N=21
(%)Placebo
N=24
(%)- *
- Reported in at least 2 PERTZYE-treated patients (≥10%) and at a higher rate than placebo-treated patients
Diarrhea 10% 4% Dyspepsia 10% 4% Cough 10% 4% 6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of PERTZYE or other pancreatic enzyme products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Eye Disorders
- blurred vision
Gastrointestinal Disorders
- fibrosing colonopathy, distal intestinal obstruction syndrome
- abdominal pain, flatulence, constipation, and nausea
Immune System Disorders
- anaphylaxis, asthma, hives, and pruritus
Investigations
- asymptomatic elevations of liver enzymes
Musculoskeletal System
- myalgia, muscle spasm
Skin and Subcutaneous Tissue Disorders
- urticaria and rash
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Published data from case reports with pancrelipase use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. Pancrelipase is minimally absorbed systematically; therefore, maternal use is not expected to result in fetal exposure to the drug. Animal reproduction studies have not been conducted with pancrelipase.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of pancrelipase in either human or animal milk, the effects on the breastfed infant or the effects on milk production. Pancrelipase is minimally absorbed systemically following oral administration, therefore maternal use is not expected to result in clinically relevant exposure of breastfed infants to the drug. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for PERTZYE and any potential adverse effects on the breastfed infant from PERTZYE or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of PERTZYE for the treatment of exocrine pancreatic insufficiency have been established in pediatric patients.
Use of PERTZYE for this indication is supported by a randomized, double-blind, placebo-controlled, crossover study of 24 pediatric patients, 8 years and older with exocrine pancreatic insufficiency due to cystic fibrosis. The safety in pediatric patients was similar to that observed in adult patients [see Adverse Reactions (6.1) and Clinical Studies (14)].
Dosages exceeding 6,000 lipase units/kg/meal have been reported postmarketing to be associated with fibrosing colonopathy and colonic strictures in pediatric patients less than 12 years of age. If there is a history of fibrosing colonopathy, monitor patients during treatment with PERTZYE because some patients may be at risk of progressing to stricture formation. Do not exceed the recommended dosage of either 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or 4,000 lipase units/g fat ingested/day in pediatric patients greater than 12 months of age without further investigation [see Dosage and Administration (2.1) and Warnings and Precautions (5.1)].
Crushing or chewing PERTZYE capsules or mixing the capsule contents in foods having a pH greater than 4.5 can disrupt the protective enteric coating on the capsule contents and result in early release of enzymes, irritation of the oral mucosa, and/or loss of enzyme activity. Instruct the patient or caregiver of the following: consume sufficient liquids (juice, water, breast milk, or formula) to ensure complete swallowing, and visually inspect the mouth of pediatric patients less than 12 months of age to ensure that no drug is retained in the mouth and irritation of the oral mucosa has not occurred [see Dosage and Administration (2.3) and Warnings and Precautions (5.2)].
8.5 Geriatric Use
Clinical studies of PERTZYE did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between patients aged 65 years and over and younger adult patients.
-
10 OVERDOSAGE
Chronic high dosages of pancreatic enzyme products have been associated with fibrosing colonopathy and colonic strictures [see Warnings and Precautions (5.1)]. High dosages of pancreatic enzyme products have been associated with hyperuricosuria and hyperuricemia [see Warnings and Precautions (5.3)].
-
11 DESCRIPTION
Pancrelipase is a pancreatic enzyme product consisting of a mixture of enzymes including lipases, proteases, and amylases, and is an extract derived from porcine pancreatic glands. The enteric-coated microspheres in PERTZYE are formulated to release pancreatic enzymes at an approximate pH of 5.5 or greater.
PERTZYE (pancrelipase) delayed-release capsules are for oral administration, include a two-piece hard gelatin shell containing light tan/cream-colored bicarbonate-buffered enteric-coated microspheres ranging in size from 0.8 to 1.4 mm in diameter for 4,000 USP units of lipase and 0.8 to 2.2 mm in diameter for 8,000, 16,000, and 24,000 USP units of lipase, and are available as follows:
4,000 USP units of lipase; 14,375 USP units of protease; and 15,125 USP units of amylase; delayed-release capsules have a clear body printed in green with "4" and a clear cap printed with a green circular stripe and "DCI". The imprinting ink on the capsule contains FD&C Blue #1, D&C Yellow #10, black iron oxide, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, shellac, and ammonium hydroxide.
8,000 USP units of lipase; 28,750 USP units of protease; and 30,250 USP units of amylase; delayed-release capsules have a clear body printed in blue with "8" and a clear cap printed with a blue circular stripe and "DCI". The imprinting ink on the capsule contains FD&C Blue #1, ethanol, methanol, n-butyl alcohol, propylene glycol, shellac, and ammonium hydroxide.
16,000 USP units of lipase; 57,500 USP units of protease; and 60,500 USP units of amylase; delayed-release capsules have a clear body printed in red with "16" and a clear cap printed with a red circular stripe and "DCI". The imprinting ink on the capsule contains FD&C Red #40, povidone, titanium dioxide, dehydrated alcohol, sodium hydroxide, butyl alcohol, propylene glycol, isopropyl alcohol, and shellac.
24,000 USP units of lipase; 86,250 USP units of protease; and 90,750 USP units of amylase; delayed-release capsules have a clear body printed in purple with "24" and a clear cap printed with a purple circular stripe and "DCI". The imprinting ink on the capsule contains FD&C Blue #2, D&C Red #7, titanium dioxide, strong ammonia solution, propylene glycol, butyl alcohol, isopropyl alcohol, dehydrated alcohol, and shellac.
PERTZYE (pancrelipase) delayed-release capsules include the following inactive ingredients: cellulose acetate phthalate, diethyl phthalate, polyvinylpyrrolidone, sodium bicarbonate, sodium carbonate, sodium starch glycolate, talc, and ursodiol.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pancreatic enzyme products contain a mixture of lipases, proteases, and amylases that catalyze the hydrolysis of fats to monoglyceride, glycerol and free fatty acids, proteins into peptides and amino acids, and starches into dextrins and short chain sugars such as maltose and maltotriose in the duodenum and proximal small intestine, thereby acting like digestive enzymes physiologically secreted by the pancreas.
12.2 Pharmacodynamics
For patients consuming a high fat diet in the clinical trials, the coefficient of fat absorption (CFA) was higher in patients who received PERTZYE compared to the placebo treatment group, indicating improved fat absorption [see Clinical Studies (14)].
-
14 CLINICAL STUDIES
Adult and Pediatric Patients
A randomized, double-blind, placebo-controlled, crossover study was conducted in 24 patients aged 8 to 43 years (mean age of 20 years) with exocrine pancreatic insufficiency due to cystic fibrosis. Patients were randomized to receive PERTZYE at individually titrated doses (not to exceed 2,500 lipase units/kg/meal) or matching placebo for 6 to 8 days of treatment, followed by crossover to the alternate treatment for an additional 6 to 8 days. The mean lipase dosage during the treatment period was 1,565 lipase units/kg/meal and a controlled high-fat diet (approximately 2g fat/kg/day) was maintained throughout the study. The length of exposure to PERTZYE during this study was 20 to 28 days, including the treatment period of 6 to 8 days, and the open label titration and transition periods of 7 to 10 days each.
The efficacy analysis population included 21 patients who completed both double-blind treatment periods.
Coefficient of Fat Absorption Endpoint and Results
The primary efficacy endpoint was the mean difference in coefficient of fat absorption (CFA) between PERTZYE and placebo treatment. The CFA was determined by a 72-hour stool collection during both treatments, when both fat ingestion and excretion were measured.
Mean CFA was 83% with PERTZYE treatment compared to 46% with placebo treatment. The mean difference in CFA was 36 percentage points in favor of PERTZYE treatment with 95% CI: (28, 45) and p<0.001.
Coefficient of Nitrogen Absorption Endpoint and Results
The coefficient of nitrogen absorption (CNA) was determined by a 72-hour stool collection during both treatments, when nitrogen excretion was measured and nitrogen ingestion from a controlled diet was estimated (based on the assumption that proteins contain 16% nitrogen). Each patient's CNA during placebo treatment was used as their no-treatment CNA value.
Mean CNA was 79% with PERTZYE treatment compared to 47% with placebo treatment. The mean difference in CNA was 32 percentage points in favor of PERTZYE treatment and this was a statistically significant change.
There were no differences between pediatric patients and adults in the severity of pancreatic insufficiency (placebo response) or in the magnitude of the response to PERTZYE.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
PERTZYE (pancrelipase) delayed-release capsules, containing light tan/cream-colored delayed-release microspheres are supplied as follows:
Strength Description Supplied As NDC Number 4,000 USP units of lipase;
14,375 USP units of protease;
15,125 USP units of amylaseTwo-piece hard gelatin capsule with a clear body printed in green with "4" and a clear cap printed with a green circular stripe and "DCI" Bottles of 100 59767-004-01 8,000 USP units of lipase;
28,750 USP units of protease;
30,250 USP units of amylaseTwo-piece hard gelatin capsule with a clear body printed in blue with "8" and a clear cap printed with a blue circular stripe and "DCI" Bottles of 100
Bottles of 25059767-008-01
59767-008-0216,000 USP units of lipase;
57,500 USP units of protease;
60,500 USP units of amylaseTwo-piece hard gelatin capsule with a clear body printed in red with "16" and a clear cap printed with a red circular stripe and "DCI" Bottles of 100
Bottles of 25059767-016-01
59767-016-0224,000 USP units of lipase;
86,250 USP units of protease;
90,750 USP units of amylaseTwo-piece hard gelatin capsule with a clear body printed in purple with "24" and a clear cap printed with a purple circular stripe and "DCI" Bottles of 80
Bottles of 20059767-024-01
59767-024-02Storage and Handling
- Store PERTZYE at room temperature 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to 40°C (59°F to 104°F) for up to 30 days. PERTZYE hard gelatin capsules should be stored in a dry place in the original container or equivalent airtight container. After opening, keep the container tightly closed between uses to protect from moisture.
- PERTZYE is dispensed in bottles containing a desiccant.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Fibrosing Colonopathy
Advise the patient or caregiver that fibrosing colonopathy has been reported with high dosages of pancreatic enzyme products, usually with use over a prolonged period of time and in pediatric patients less than 12 years of age. Advise patients and caregivers that if signs and symptoms of colon stricture formation occur (e.g., stomach area (abdominal) pain, bloating, trouble passing stool (constipation), nausea, vomiting, diarrhea) to immediately contact their healthcare provider [see Warnings and Precautions (5.1)].
Hyperuricemia
Advise the patient or caregiver that hyperuricemia may occur in patients with gout or renal impairment and to contact the healthcare provider if they experience pain, stiffness, redness or swelling of their joints [see Warnings and Precautions (5.3)].
Hypersensitivity Reactions
Inform the patient or caregiver that severe hypersensitivity reactions, including anaphylaxis, asthma, hives, and pruritus, have been reported with use of pancreatic enzyme products. Seek medical attention if signs or symptoms of a hypersensitivity reaction develop [see Warnings and Precautions (5.5)].
Dosage
Advise the patient or caregiver to take or administer PERTZYE as prescribed, and to contact the healthcare provider if signs and symptoms of malabsorption persist [see Dosage and Administration (2.2)].
Administration
Instruct the patient or caregiver to:
- Take PERTZYE with meals or snacks.
- Swallow capsules whole.
- For patients unable to swallow intact capsules:
- the capsule contents may be sprinkled on a small amount of soft acidic food with a pH of 4.5 or less (e.g., applesauce). The 4,000 USP lipase unit capsule may also be administered with applesauce via gastrostomy tube 14 French or larger.
- For pediatric patients birth to 12 months of age, PERTZYE capsules can be opened, and the capsule contents sprinkled directly into the infant's mouth.
- See the Instructions for Use for a description of all preparation and administration instructions.
- Consume sufficient liquids (juice, water, breast milk, or formula) and visually inspect an infant's mouth to ensure complete swallowing of PERTZYE capsules or capsule contents [see Warnings and Precautions (5.2)].
- Do not crush or chew PERTZYE capsules or capsule contents.
- Do not mix the PERTZYE capsule contents directly into a bottle of breast milk or formula.
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 02/2024 MEDICATION GUIDE
PERTZYE (pert-zye)
(pancrelipase)
delayed-release capsulesWhat is the most important information I should know about PERTZYE?
PERTZYE may increase the risk of having a rare bowel disorder called fibrosing colonopathy, especially if taken at a high dose for a long time in children with cystic fibrosis. This condition is serious and may require surgery. The risk of having this condition may be reduced by following the dosing instructions that your doctor gives you.
Call your doctor right away if you have any unusual or severe:- stomach area (abdominal) pain
- bloating
- trouble passing stool (constipation)
- nausea
- vomiting
- diarrhea
Take PERTZYE exactly as prescribed by your doctor. Do not take more PERTZYE than directed by your doctor. What is PERTZYE? - PERTZYE is a prescription medicine used to treat people who cannot digest food normally because their pancreas does not make enough enzymes.
- PERTZYE capsules contain a mixture of digestive enzymes including lipases, proteases and amylases from pig pancreas.
- PERTZYE is safe and effective in adults and children.
Before taking PERTZYE, tell your doctor about all of your medical conditions, including if you: - are allergic to pork (pig) products.
- have a history of blockage of your intestines or scarring or thickening of your bowel wall (fibrosing colonopathy).
- have gout, kidney disease, or high blood uric acid (hyperuricemia).
- have trouble swallowing capsules.
- have any other medical condition.
- are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if PERTZYE passes into your breast milk. Talk to your doctor about the best way to feed your baby if you take PERTZYE.
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.How should I take PERTZYE? - See the Instructions for Use at the end of this Medication Guide for detailed instructions about how to give PERTZYE by mouth and through a gastrostomy tube.
- Take PERTZYE capsules exactly as your doctor tells you. Contact your doctor if you continue to have signs and symptoms of malabsorption (not absorbing nutrients from food) such as abdominal pain, abdominal bloating, fatty stools, or weight loss. Your dose may need to be changed.
- You should not switch PERTZYE with any other pancreatic enzyme product without first talking with your doctor.
- Do not take more capsules in a day than the number your doctor tells you to take (total daily dose).
- Always take PERTZYE with a meal or snack. If you eat a lot of meals or snacks in a day, be careful not to go over your total daily dose.
- Your doctor may change your dose based on the amount of fatty foods you eat or based on your weight.
- PERTZYE capsules should be swallowed whole. Do not crush or chew the PERTZYE capsules or their contents and do not hold the capsule or capsule contents in your mouth. Crushing or chewing the PERTZYE capsules may cause irritation in your mouth or change the way PERTZYE works in your body.
- Drink plenty of liquid to ensure complete swallowing of the PERTZYE capsules or PERTZYE capsule contents-food mixture.
- Throw away the PERTZYE capsule contents-food mixture that is not used. Do not save this mixture for later use.
- If you miss a dose of PERTZYE, wait until your next meal or snack and take your prescribed dose. Take your next dose at your usual time. Do not take two doses at one time.
What are the possible side effects of PERTZYE? - See "What is the most important information I should know about PERTZYE?"
- Irritation of the inside of your mouth. This can happen if PERTZYE is not swallowed completely, is crushed or chewed, or is mixed in foods that are not recommended.
- Increase in blood uric acid levels (hyperuricemia). This may happen in people with gout, kidney problems, or those who take high doses of pancrelipase, the active ingredient in PERTZYE. Call your doctor if you have pain, stiffness, redness or swelling in your joints.
- Severe allergic reactions. Severe allergic reactions have happened in people taking pancreatic enzyme products like PERTZYE. Stop taking PERTZYE and get emergency treatment right away if you have any of these symptoms: trouble with breathing, skin rashes, swollen lips or itching.
The most common side effects of PERTZYE include: - diarrhea
- upset stomach (indigestion)
- cough
Other possible side effects:
PERTZYE and other pancreatic enzyme products are made from the pancreas of pigs, the same pigs people eat as pork. These pigs may carry viruses. Although it has never been reported, it may be possible for a person to get a viral infection from taking pancreatic enzyme products that come from pigs.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of PERTZYE. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store PERTZYE? - Store PERTZYE at room temperature between 68ºF to 77ºF (20°C to 25°C).
- Keep PERTZYE in a dry place and in the original container or equivalent airtight container.
- After opening the bottle, keep it tightly closed between uses to keep your medicine dry (protect it from moisture).
- The PERTZYE bottle contains a desiccant packet to help keep your medicine dry. Do not eat or throw away the desiccant packet in your medicine bottle.
General information about the safe and effective use of PERTZYE.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use PERTZYE for a condition for which it was not prescribed. Do not give PERTZYE to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about PERTZYE that is written for health professionals.What are the ingredients in PERTZYE?
Active ingredients: lipase, protease, and amylase.
Inactive ingredients: cellulose acetate phthalate, diethyl phthalate, polyvinylpyrrolidone, sodium bicarbonate, sodium carbonate, sodium starch glycolate, talc, and ursodiol.
The hard gelatin capsule shells contain: gelatin and water.
The green imprinting ink on the 4,000 units of lipase; 14,375 units of protease; 15,125 units of amylase capsules contain: FD&C Blue #1, D&C Yellow #10, black iron oxide, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, shellac and ammonium hydroxide.
The blue imprinting ink on the 8,000 units of lipase; 28,750 units of protease; 30,250 units of amylase capsules contain: FD&C Blue #1, ethanol, methanol, n-butyl alcohol, propylene glycol, shellac and ammonium hydroxide.
The red imprinting ink on the 16,000 units of lipase; 57,500 units of protease; 60,500 units of amylase capsules contain: FD&C Red #40, povidone, titanium dioxide, dehydrated alcohol, sodium hydroxide, butyl alcohol, propylene glycol, isopropyl alcohol, and shellac.
The purple imprinting ink on the 24,000 units of lipase; 86,250 units of protease; 90,750 units of amylase capsules contain: FD&C Blue #2, D&C Red #7, titanium dioxide, strong ammonia solution, propylene glycol, butyl alcohol, isopropyl alcohol, dehydrated alcohol, and shellac.
For more information, go to www.Pertzye.com or call 1-877-882-5950.Manufactured by:
Digestive Care, Inc.
1120 Win Drive
Bethlehem, PA 18017
U.S. License No. 2184
Product of USA -
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
PERTZYE (pert-zye)
(pancrelipase)
delayed-release capsulesThis Instructions for Use contains information on how to give PERTZYE by mouth and through a gastrostomy tube.
Important Information You Need to Know Before Taking PERTZYE:
- Always take PERTZYE with a meal or snack.
- PERTZYE capsules should be swallowed whole. Do not crush or chew the PERTZYE capsules or their contents, and do not hold the capsule or capsule contents in your mouth.
- Drink plenty of liquid to ensure complete swallowing of PERTZYE capsules or PERTZYE capsule contents-food mixture.
- Throw away the PERTZYE capsule contents-food mixture that is not used. Do not save this mixture for later use.
Giving PERTZYE to infants (Children from Birth to 12 Months of Age) by mouth:
The two methods to give PERTZYE to Infants (Children from Birth to 12 Months of Age) are described below:
a) Giving with Applesauce Before Breast or Formula Feeding
- Place about 2 teaspoons of applesauce, the kind found in baby food jars that you buy at the store, or other food recommended by your doctor, into a clean container.
- Carefully open the PERTZYE capsule by grasping and slightly squeezing both ends of the capsule and then twisting and pulling the capsule apart. Pour the capsule contents onto the food in the clean container. Throw away the empty PERTZYE capsule.
- Carefully mix the capsule contents evenly through the food. Be careful not to crush the PERTZYE capsule contents when mixing.
- Give the PERTZYE capsule contents-food mixture to your infant right away.
- Give your infant 4 ounces (120 mL) of formula or breastfeed your infant right away.
- Look in your infant's mouth to make sure that all of the medicine is swallowed and there is no PERTZYE capsule contents-food mixture left in your infant's mouth.
b) Giving Directly into the Infant's Mouth Before Breast or Formula Feeding
- Carefully open the PERTZYE capsule by grasping and slightly squeezing both ends of the capsule and then twisting and pulling the capsule apart. Empty the capsule contents into your infant's mouth.
- Give your infant 4 ounces (120 mL) of formula or breastfeed right away.
- Look in your infant's mouth to make sure that all of the medicine is swallowed and there is no PERTZYE capsule contents left in your infant's mouth.
- Throw away the empty PERTZYE capsule.
Do not mix the PERTZYE capsule contents directly into a bottle of breast milk or formula.
Giving PERTZYE to children and adults by mouth:
- Swallow PERTZYE capsules whole and take them with enough liquid to swallow them right away.
- If you have trouble swallowing capsules, follow these instructions for taking PERTZYE with food:
- Place about 2 teaspoons of applesauce, or other food recommended by your doctor, into a clean container.
- Carefully open the capsules that you will need for the prescribed dose by grasping and slightly squeezing both ends of the capsule and then twisting and pulling the capsule apart. Pour the capsule contents onto the food in the clean container.
- Throw away the empty PERTZYE capsules.
- Carefully mix the capsule contents evenly through the food. Be careful not to crush the PERTZYE capsule contents when mixing.
- Swallow all of the PERTZYE capsule contents-food mixture right away.
- Drink plenty of water or juice to make sure that all of the medicine is swallowed and there is no PERTZYE capsule contents-food mixture left in the mouth.
Giving PERTZYE through a gastrostomy tube:
- PERTZYE 4,000 lipase units capsule contents may be given through a gastrostomy tube that is size 14 French or larger. Do not give PERTZYE through a gastrostomy tube smaller than size 14 French.
- Do not give more than the contents of 2 PERTZYE 4,000 lipase units capsules through a gastrostomy tube at a time.
- Give the PERTZYE capsule contents-food mixture right away after it is prepared.
- 1.
- Place at least 2 teaspoons of applesauce, or other food recommended by your doctor, into a clean container.
- 2.
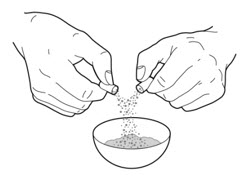
- Carefully open 1 or 2 of the PERTZYE capsules, depending on your prescribed dose, by grasping and slightly squeezing both ends of the capsule and then twisting and pulling the capsule apart. Pour the capsule contents onto the food in the clean container. Throw away the empty PERTZYE capsules (See Figure A).
- 3.
- Carefully mix the capsule contents evenly through the food. Be careful not to crush the PERTZYE capsule contents when mixing.
- 4.
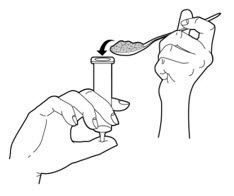
- Remove the plunger from a 35 mL slip tip syringe. Cover the tip of the syringe with your finger.
- 5.
- Carefully spoon the PERTZYE capsule contents-food mixture into the slip tip syringe (See Figure B).
- 6.
- Replace the plunger partially back into the slip tip syringe.
- 7.
- Turn the slip tip syringe so the syringe tip is facing upward. Remove your finger from the tip of the slip tip syringe.
- 8.
- Gently shake or tap the slip tip syringe so that the PERTZYE capsule contents-food mixture moves down towards the plunger.
- 9.
- Carefully push up slowly on the plunger until the PERTZYE capsule contents-food mixture fills the syringe tip (See Figure C).
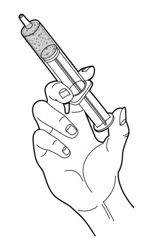
- 10.
- Connect the slip tip syringe directly into the gastrostomy tube feeding port.
- 11.
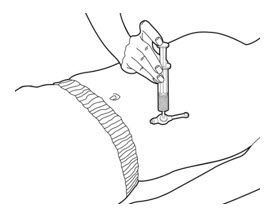
- Press on the plunger of the slip tip syringe using steady pressure to push all of the PERTZYE capsule contents-food mixture into the gastrostomy tube feeding port over 10 seconds to 12 seconds (See Figure D).
- 12.
- Flush the gastrostomy tube feeding port with about 10 mL of water.
- 13.
- If your prescribed dose is more than 2 capsules of PERTZYE, repeat steps 1 through 12, using only 1 or 2 PERTZYE capsules at a time, until the prescribed dose is given.
Storing PERTZYE:
- Store PERTZYE at room temperature between 68ºF to 77ºF (20°C to 25°C).
- Keep PERTZYE in a dry place and in the original container or equivalent airtight container.
- After opening the bottle, keep it tightly closed between uses to keep your medicine dry (protect it from moisture).
- The PERTZYE bottle contains a desiccant packet to help keep your medicine dry. Do not eat or throw away the desiccant packet in your medicine bottle.
Keep PERTZYE and all medicines out of the reach of children.
Manufactured by:
Digestive Care, Inc.
1120 Win Drive
Bethlehem, PA 18017
U.S. License No. 2184
Product of USA
© 2024 Digestive Care, Inc.This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 02/2024 -
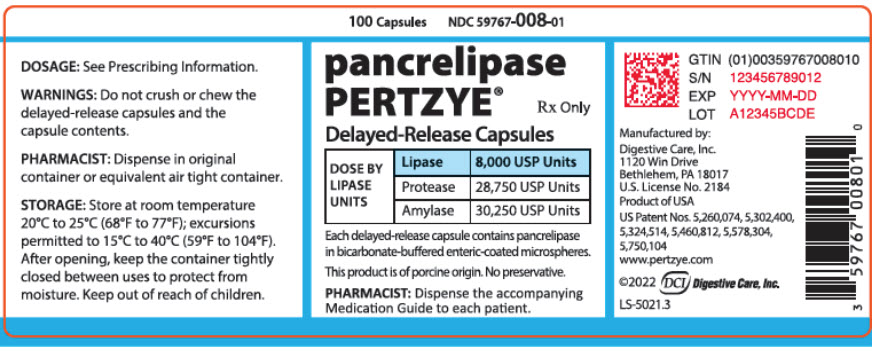
PRINCIPAL DISPLAY PANEL - 100 Capsule Bottle Label - 59767-008-01
100 Capsules
NDC 59767-008-01pancrelipase
PERTZYE®Rx Only
Delayed-Release Capsules
DOSE BY
LIPASE
UNITSLipase
8,000 USP UnitsProtease
28,750 USP UnitsAmylase
30,250 USP UnitsEach delayed-release capsule contains pancrelipase
in bicarbonate-buffered enteric-coated microspheres.This product is of porcine origin. No preservative.
PHARMACIST: Dispense the accompanying
Medication Guide to each patient.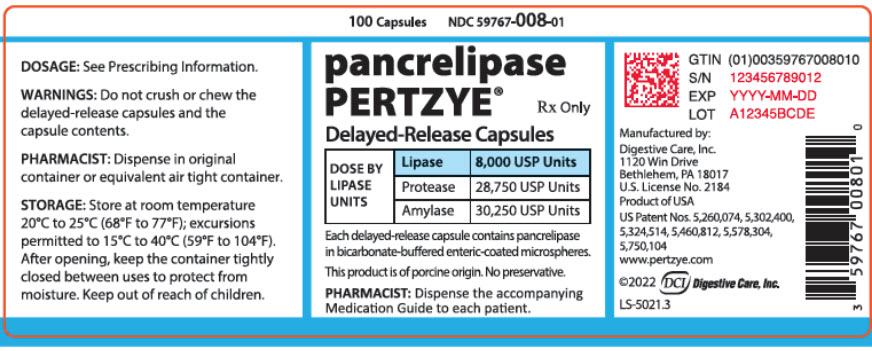
-
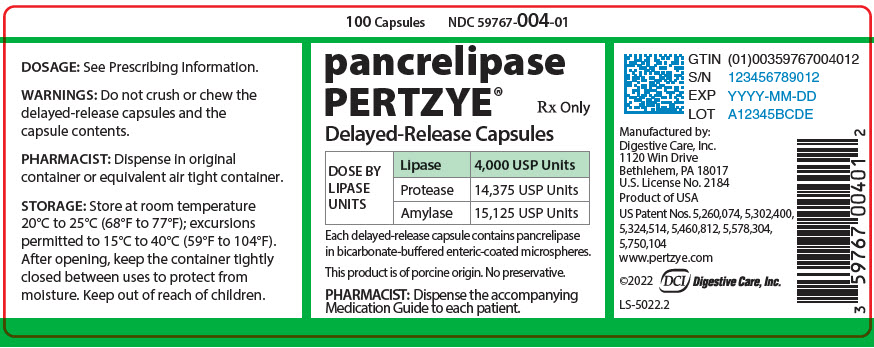
PRINCIPAL DISPLAY PANEL - 100 Capsule Bottle Label - 59767-004-01
100 Capsules
NDC 59767-004-01pancrelipase
PERTZYE®Rx Only
Delayed-Release Capsules
DOSE BY
LIPASE
UNITSLipase
4,000 USP UnitsProtease
14,375 USP UnitsAmylase
15,125 USP UnitsEach delayed-release capsule contains pancrelipase
in bicarbonate-buffered enteric-coated microspheres.This product is of porcine origin. No preservative.
PHARMACIST: Dispense the accompanying
Medication Guide to each patient.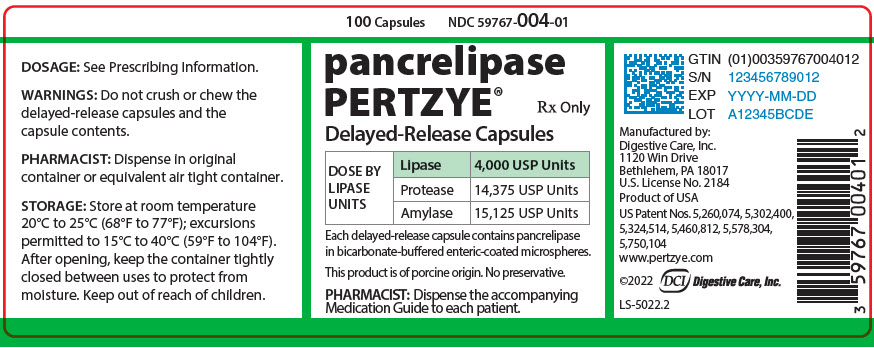
-
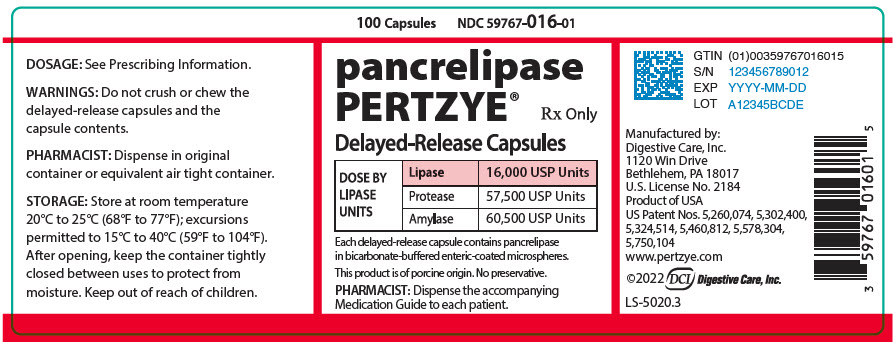
PRINCIPAL DISPLAY PANEL - 100 Capsule Bottle Label - 59767-016-01
100 Capsules
NDC 59767-016-01pancrelipase
PERTZYE®Rx Only
Delayed-Release Capsules
DOSE BY
LIPASE
UNITSLipase
16,000 USP UnitsProtease
57,500 USP UnitsAmylase
60,500 USP UnitsEach delayed-release capsule contains pancrelipase
in bicarbonate-buffered enteric-coated microspheres.This product is of porcine origin. No preservative.
PHARMACIST: Dispense the accompanying
Medication Guide to each patient.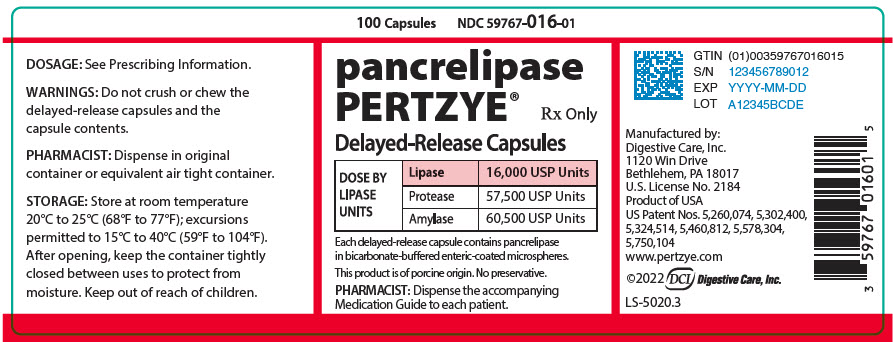
-
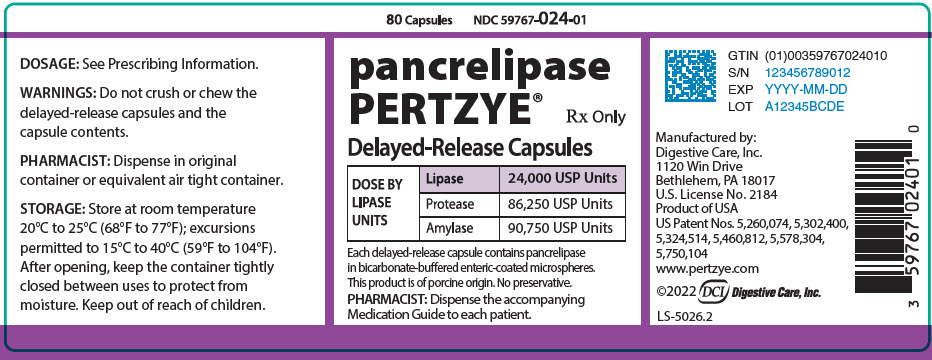
PRINCIPAL DISPLAY PANEL - 80 Capsule Bottle Label
80 Capsules
NDC 59767-024-01pancrelipase
PERTZYE®Rx Only
Delayed-Release Capsules
DOSE BY
LIPASE
UNITSLipase
24,000 USP UnitsProtease
86,250 USP UnitsAmylase
90,750 USP UnitsEach delayed-release capsule contains pancrelipase
in bicarbonate-buffered enteric-coated microspheres.This product is of porcine origin. No preservative.
PHARMACIST: Dispense the accompanying
Medication Guide to each patient.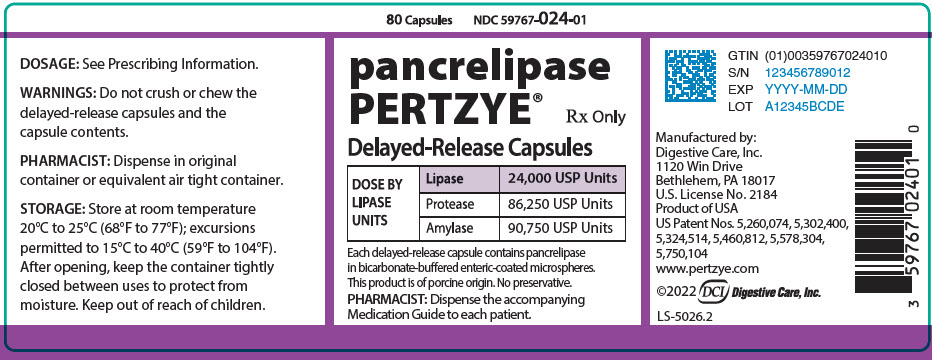
-
INGREDIENTS AND APPEARANCE
PERTZYE
pancrelipase capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59767-008 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANCRELIPASE LIPASE (UNII: 8MYC33932O) (PANCRELIPASE LIPASE - UNII:8MYC33932O) PANCRELIPASE LIPASE 8000 [USP'U] PANCRELIPASE AMYLASE (UNII: YOJ58O116E) (PANCRELIPASE AMYLASE - UNII:YOJ58O116E) PANCRELIPASE AMYLASE 30250 [USP'U] PANCRELIPASE PROTEASE (UNII: 3560D81V50) (PANCRELIPASE PROTEASE - UNII:3560D81V50) PANCRELIPASE PROTEASE 28750 [USP'U] Inactive Ingredients Ingredient Name Strength CELLACEFATE (UNII: F2O5O2OI9F) DIETHYL PHTHALATE (UNII: UF064M00AF) POVIDONE K90 (UNII: RDH86HJV5Z) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM CARBONATE (UNII: 45P3261C7T) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) TALC (UNII: 7SEV7J4R1U) URSODIOL (UNII: 724L30Y2QR) Product Characteristics Color blue (Clear capsule shell with blue band) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code DCI;8 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59767-008-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/17/2012 2 NDC:59767-008-02 250 in 1 BOTTLE; Type 0: Not a Combination Product 05/17/2012 3 NDC:59767-008-99 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/17/2012 4 NDC:59767-008-00 20 in 1 BOTTLE; Type 0: Not a Combination Product 01/23/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022175 05/17/2012 PERTZYE
pancrelipase capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59767-004 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANCRELIPASE LIPASE (UNII: 8MYC33932O) (PANCRELIPASE LIPASE - UNII:8MYC33932O) PANCRELIPASE LIPASE 4000 [USP'U] PANCRELIPASE AMYLASE (UNII: YOJ58O116E) (PANCRELIPASE AMYLASE - UNII:YOJ58O116E) PANCRELIPASE AMYLASE 15125 [USP'U] PANCRELIPASE PROTEASE (UNII: 3560D81V50) (PANCRELIPASE PROTEASE - UNII:3560D81V50) PANCRELIPASE PROTEASE 14375 [USP'U] Inactive Ingredients Ingredient Name Strength CELLACEFATE (UNII: F2O5O2OI9F) DIETHYL PHTHALATE (UNII: UF064M00AF) POVIDONE K90 (UNII: RDH86HJV5Z) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM CARBONATE (UNII: 45P3261C7T) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) TALC (UNII: 7SEV7J4R1U) URSODIOL (UNII: 724L30Y2QR) Product Characteristics Color green (Clear capsule shell with green band) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code DCI;4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59767-004-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/06/2016 2 NDC:59767-004-99 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/06/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022175 10/06/2016 PERTZYE
pancrelipase capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59767-016 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANCRELIPASE LIPASE (UNII: 8MYC33932O) (PANCRELIPASE LIPASE - UNII:8MYC33932O) PANCRELIPASE LIPASE 16000 [USP'U] PANCRELIPASE AMYLASE (UNII: YOJ58O116E) (PANCRELIPASE AMYLASE - UNII:YOJ58O116E) PANCRELIPASE AMYLASE 60500 [USP'U] PANCRELIPASE PROTEASE (UNII: 3560D81V50) (PANCRELIPASE PROTEASE - UNII:3560D81V50) PANCRELIPASE PROTEASE 57500 [USP'U] Inactive Ingredients Ingredient Name Strength CELLACEFATE (UNII: F2O5O2OI9F) DIETHYL PHTHALATE (UNII: UF064M00AF) POVIDONE K90 (UNII: RDH86HJV5Z) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM CARBONATE (UNII: 45P3261C7T) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) TALC (UNII: 7SEV7J4R1U) URSODIOL (UNII: 724L30Y2QR) Product Characteristics Color red (Clear capsule shell with red band) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code DCI;16 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59767-016-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/17/2012 2 NDC:59767-016-02 250 in 1 BOTTLE; Type 0: Not a Combination Product 05/17/2012 3 NDC:59767-016-99 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/17/2012 4 NDC:59767-016-00 20 in 1 BOTTLE; Type 0: Not a Combination Product 01/23/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022175 05/17/2012 PERTZYE
pancrelipase capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59767-024 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANCRELIPASE LIPASE (UNII: 8MYC33932O) (PANCRELIPASE LIPASE - UNII:8MYC33932O) PANCRELIPASE LIPASE 24000 [USP'U] PANCRELIPASE AMYLASE (UNII: YOJ58O116E) (PANCRELIPASE AMYLASE - UNII:YOJ58O116E) PANCRELIPASE AMYLASE 90750 [USP'U] PANCRELIPASE PROTEASE (UNII: 3560D81V50) (PANCRELIPASE PROTEASE - UNII:3560D81V50) PANCRELIPASE PROTEASE 86250 [USP'U] Inactive Ingredients Ingredient Name Strength CELLACEFATE (UNII: F2O5O2OI9F) DIETHYL PHTHALATE (UNII: UF064M00AF) POVIDONE K90 (UNII: RDH86HJV5Z) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM CARBONATE (UNII: 45P3261C7T) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) TALC (UNII: 7SEV7J4R1U) URSODIOL (UNII: 724L30Y2QR) Product Characteristics Color purple (Clear capsule shell with purple band) Score no score Shape CAPSULE Size 25mm Flavor Imprint Code DCI;24 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59767-024-01 80 in 1 BOTTLE; Type 0: Not a Combination Product 07/13/2017 2 NDC:59767-024-02 200 in 1 BOTTLE; Type 0: Not a Combination Product 07/13/2017 3 NDC:59767-024-99 80 in 1 BOTTLE; Type 0: Not a Combination Product 07/13/2017 4 NDC:59767-024-00 20 in 1 BOTTLE; Type 0: Not a Combination Product 07/13/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022175 07/13/2017 Labeler - Digestive Care, Inc. (831065214) Establishment Name Address ID/FEI Business Operations Digestive Care, Inc. 831065214 manufacture(59767-008, 59767-016, 59767-004, 59767-024)