Label: EVENITY- romosozumab-aqqg injection, solution
- NDC Code(s): 55513-880-01, 55513-880-02, 55513-998-01, 55513-998-02
- Packager: Amgen Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated April 14, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EVENITY safely and effectively. See full prescribing information for EVENITY. EVENITY® (romosozumab-aqqg) injection, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: POTENTIAL RISK OF MYOCARDIAL INFARCTION, STROKE AND CARDIOVASCULAR DEATH
- EVENITY may increase the risk of myocardial infarction, stroke, and cardiovascular death [see Warnings and Precautions (5.1)]. EVENITY should not be initiated in patients who have had a myocardial infarction or stroke within the preceding year. Consider whether the benefits outweigh the risks in patients with other cardiovascular risk factors. If a patient experiences a myocardial infarction or stroke during therapy, EVENITY should be discontinued.
-
1 INDICATIONS AND USAGE1.1 Treatment of Postmenopausal Women with Osteoporosis at High Risk for Fracture - EVENITY is indicated for the treatment of osteoporosis in postmenopausal women at high risk for fracture ...
-
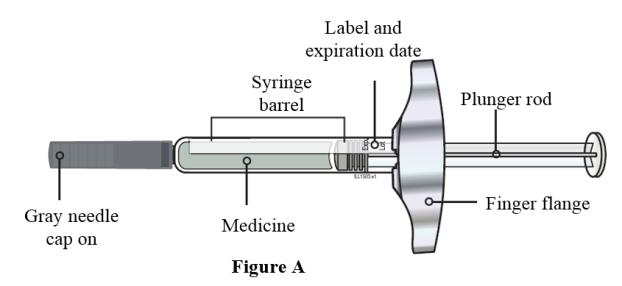
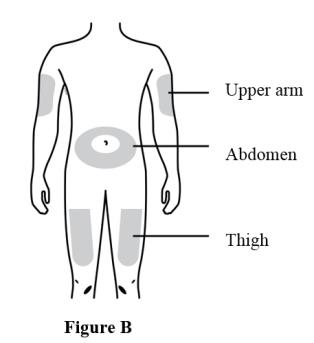
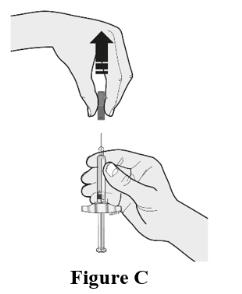
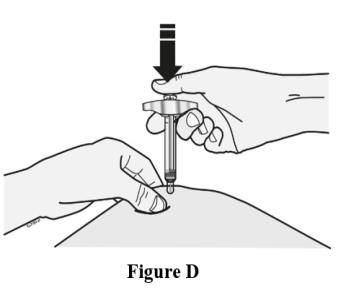
2 DOSAGE AND ADMINISTRATION2.1 Important Dosage and Administration Instructions - Two separate syringes (and two separate subcutaneous injections) are needed to administer the total dose of 210 mg of EVENITY. Inject two ...
-
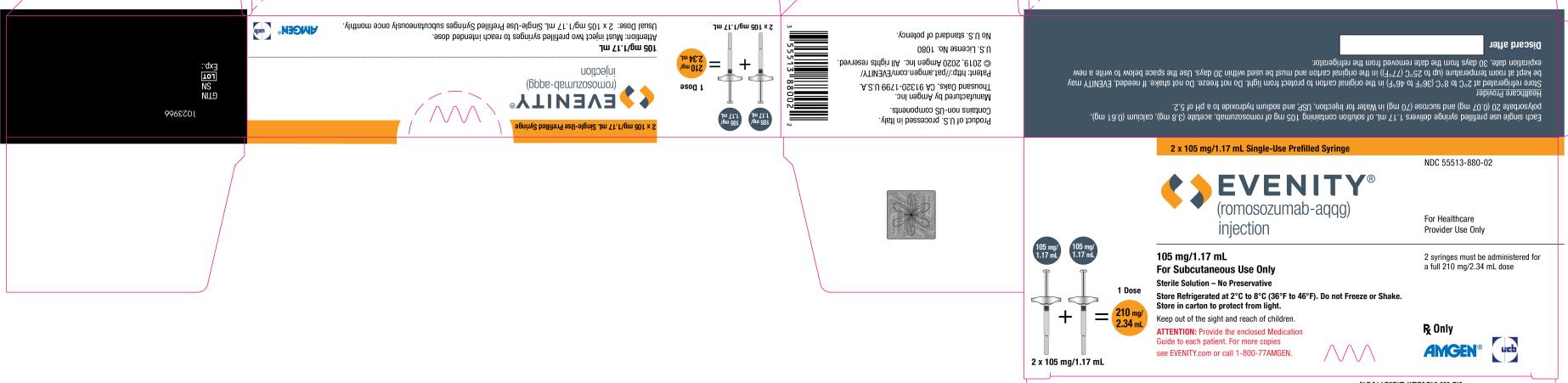
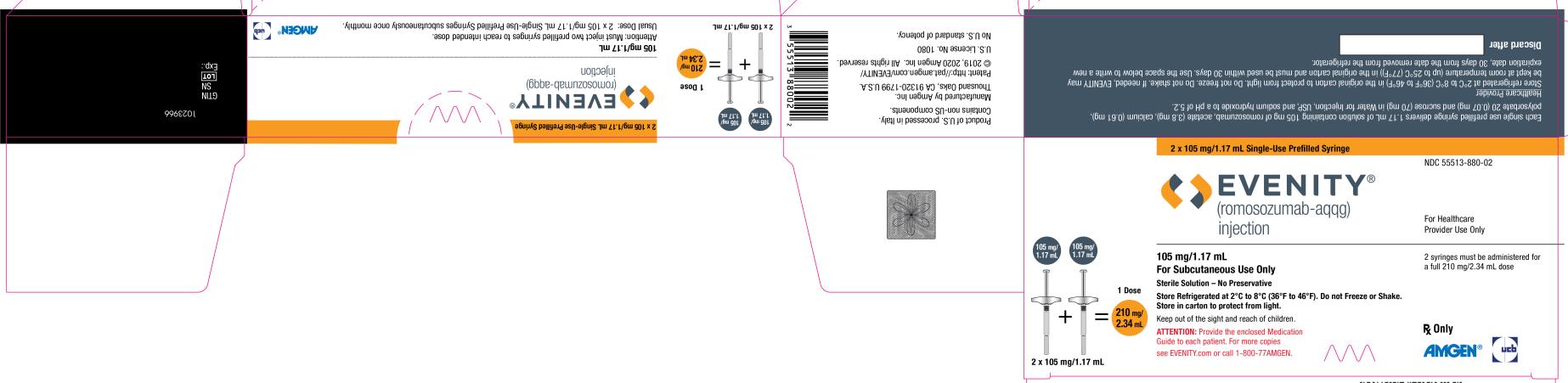
3 DOSAGE FORMS AND STRENGTHSInjection: 105 mg/1.17 mL clear to opalescent, colorless to light yellow solution in a single-use prefilled syringe. A full dose of EVENITY requires two single-use prefilled syringes.
-
4 CONTRAINDICATIONSEVENITY is contraindicated in patients with: Hypocalcemia. Pre-existing hypocalcemia must be corrected prior to initiating therapy with EVENITY [see Warnings and Precautions (5.3), Adverse ...
-
5 WARNINGS AND PRECAUTIONS5.1 Major Adverse Cardiac Events (MACE) In a randomized controlled trial in postmenopausal women, there was a higher rate of major adverse cardiac events (MACE), a composite endpoint of ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the label: Major adverse cardiac events [see Boxed Warning and Warnings and Precautions ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - EVENITY is not indicated for use in women of reproductive potential. In animal reproduction studies, weekly administration of romosozumab-aqqg to pregnant rats ...
-
11 DESCRIPTIONRomosozumab-aqqg is a humanized monoclonal antibody (IgG2) produced in a mammalian cell line (Chinese Hamster Ovary) by recombinant DNA technology that binds to and inhibits sclerostin ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - EVENITY inhibits the action of sclerostin, a regulatory factor in bone metabolism. EVENITY increases bone formation and, to a lesser extent, decreases bone resorption ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity - In a rat carcinogenicity study, once-weekly romosozumab-aqqg doses of 3, 10 or 50 mg/kg were administered by ...
-
14 CLINICAL STUDIES14.1 Treatment of Osteoporosis in Postmenopausal Women - Study 1 (NCT01575834) was a randomized, double-blind, placebo-controlled study of postmenopausal women aged 55 to 90 years (mean age ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - EVENITY (romosozumab-aqqg) injection is a clear to opalescent, colorless to light yellow solution for subcutaneous injection supplied in a single-use prefilled syringe. Each ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Major Adverse Cardiac Events - Advise patients to seek immediate medical attention if they experience signs or ...
-
SPL UNCLASSIFIED SECTIONEVENITY® (romosozumab-aqqg) Manufactured by: Amgen Inc. One Amgen Center Drive - Thousand Oaks, California 91320-1799 - U.S. License No. 1080 - Patent: http://pat.amgen.com/EVENITY/ © 2019, 2020, 2024 ...
-
MEDICATION GUIDEMEDICATION GUIDE - EVENITY® (E-ven-i-tee) (romosozumab-aqqg) Injection, for subcutaneous use - What is the most important information I should know about EVENITY? EVENITY can cause serious ...
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 2 x 105 mg/1.17 mL Single-Use Prefilled Syringe - NDC 55513-880-02 - EVENITY® (romosozumab-aqqg) injection - For Healthcare - Provider Use Only - 105 mg/1.17 mL + 105 mg/1.17 mL ...
-
PRINCIPAL DISPLAY PANEL - 105 mg/1.17 mL Syringe Carton2 x 105 mg/1.17 mL Single-Use Prefilled Syringe - NDC 55513-998-02 - EVENITY® (romosozumab-aqqg) injection - For Healthcare - Provider Use Only - 105 mg/ 1.17 mL - + 105 mg/ 1.17 mL - = 1 Dose - 210 mg/ 2.34 ...
-
INGREDIENTS AND APPEARANCEProduct Information