Label: LIDOCAINE HCL - HYDROCORTISONE ACETATE WITH ALOE- lidocaine hcl and hydrocortisone acetate gel
- NDC Code(s): 59088-817-01, 59088-817-07
- Packager: PureTek Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 9, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
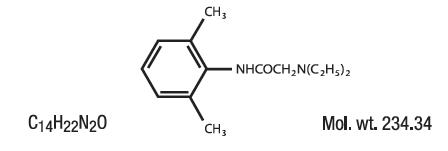
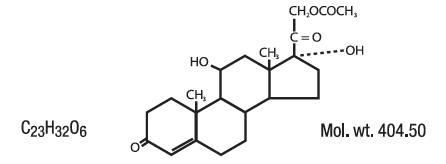
DESCRIPTION:Anti-Inflammatory Anesthetic for Relief of Hemorrhoid Pain, Swelling and Inflammation. Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), and has the ...
-
INACTIVE INGREDIENTS:ALOE BARBADENSIS (ALOE VERA) LEAF JUICE, CITRIC ACID, HYDROXYETHYL CELLULOSE, METHYLPARABEN, PEG-4, PROPYLENE GLYCOL, PROPYLPARABEN, PURIFIED WATER.
-
CLINICAL PHARMACOLOGY:MECHANISM OF ACTION: Product releases lidocaine to stabilize the neuronal membrane by inhibiting the ionic fluxes required for initiation and conduction of impulses, thereby effecting ...
-
INDICATIONS:Product is used for the anti-inflammatory and anesthetic relief of itching, pain, soreness and discomfort due to hemorrhoids, anal fissures, pruritus ani and similar conditions of the anal ...
-
CONTRAINDICATIONS:Product should not be used in patients with a history of sensitivity to any of its ingredients or adverse reactions to lidocaine or amide anesthetics, which usually do not cross-react with “caine ...
-
PRECAUTIONS:For external use only.Not for ophthalmic use.Product and used applicators could harm small children if chewed or swallowed. Keep out of reach of children. Topical formulations of ...
-
PRECAUTIONS:If irritation or sensitivity occurs or infection appears, discontinue use and institute appropriate therapy. If extensive areas are treated, the possibility of systemic absorption exists. Systemic ...
-
CARCINOGENESIS, MUTAGENESIS, AND IMPAIRMENT OF FERTILITY:Long-term animal studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of topical corticosteroids. Studies to determine mutagenicity with prednisolone ...
-
USE IN PREGNANCY:Teratogenic Effects: Pregnancy Category C Reproduction studies have been performed for lidocaine in rats at doses up to 6.6 times the human dose and have revealed no evidence of harm to the ...
-
NURSING MOTHERS:It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when this drug is administered to a nursing mother.
-
PEDIATRIC USE:Safety and efficacy in children have not been established.
-
ADVERSE REACTIONS:During or immediately following application of product, there may be transient stinging or burning from open areas of skin, or transient blanching (lightening), or erythema (redness) of the ...
-
DOSAGE AND ADMINISTRATION:Apply product to the affected area(s) twice daily or as directed by a physician. Product should not be used in excess of recommendations or for prolonged use in the anal canal. If the condition ...
-
HOW SUPPLIED:PharmaPure Rx Lidocaine HCl 2.8% - Hydrocortisone Acetate 0.55% Gel with Aloe contains one (1) Multi-use 100g tube and 15 single-use applicators. NDC 59088-817-01.
-
KEEP THIS AND ALL MEDICATIONS OUT OF REACH OF CHILDREN.Store at 20º-25ºC (68º-77ºF) [see USP Controlled Room Temperature]. Protect from freezing.
-
Tube (100 g)

-
Carton

-
INGREDIENTS AND APPEARANCEProduct Information