Label: SENSIPAR- cinacalcet hydrochloride tablet, coated
- NDC Code(s): 55513-073-30, 55513-074-30, 55513-075-30
- Packager: Amgen Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SENSIPAR safely and effectively. See full prescribing information for SENSIPAR.
SENSIPAR® (cinacalcet) tablets, for oral use
Initial U.S. Approval: 2004
INDICATIONS AND USAGE
Sensipar is a positive modulator of the calcium sensing receptor indicated for:
- Secondary Hyperparathyroidism (HPT) in adult patients with chronic kidney disease (CKD) on dialysis. (1.1)
Limitations of Use: Sensipar is not indicated for use in patients with CKD who are not on dialysis
DOSAGE AND ADMINISTRATION
- Sensipar tablets should be taken with food or shortly after a meal (2.1)
- Tablets should always be taken whole and not divided (2.1)
- Secondary HPT in patients with CKD on dialysis (2.2):
○ Starting dose is 30 mg once daily.
○ Titrate dose no more frequently than every 2 to 4 weeks through sequential doses of 30, 60, 90, 120, and 180 mg once daily as necessary to achieve targeted intact parathyroid hormone (iPTH) levels.
○ iPTH levels should be measured no earlier than 12 hours after most recent dose.
- Hypercalcemia in patients with PC or hypercalcemia in patients with primary HPT (2.3):
○ Starting dose is 30 mg twice daily.
○ Titrate dose every 2 to 4 weeks through sequential doses of 30 mg twice daily, 60 mg twice daily, 90 mg twice daily, and 90 mg three or four times daily as necessary to normalize serum calcium levels.
- Once the maintenance dose has been established, monitor serum calcium approximately monthly for patients with secondary HPT and every 2 months for patients with PC or primary HPT (2.4)
DOSAGE FORMS AND STRENGTHS
- Tablets: 30, 60, and 90 mg tablets (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
-
Hypocalcemia: Life threatening events and fatal outcomes were reported. Hypocalcemia can prolong QT interval, lower the threshold for seizures, and cause hypotension, worsening heart failure, and/or arrhythmia. Monitor serum calcium carefully for the occurrence of hypocalcemia during treatment. (2.4, 5.1)
-
Upper Gastrointestinal (GI) Bleeding: Patients with risk factors for upper GI bleeding may be at increased risk. Monitor patients and promptly evaluate and treat any suspected GI bleeding. (5.2)
-
Hypotension, Worsening Heart Failure and/or Arrhythmias: In postmarketing safety surveillance, isolated, idiosyncratic cases of hypotension, worsening heart failure, and/or arrhythmia have been reported in patients with impaired cardiac function. (5.3)
- Adynamic Bone Disease: May develop if iPTH levels are suppressed below 100 pg/mL. (5.4)
ADVERSE REACTIONS
The most common adverse reactions (i.e., ≥ 25%) associated with Sensipar were nausea and vomiting. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Amgen Medical Information at 1-800-77-AMGEN (1-800-772-6436) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
- Co-administration with a strong CYP3A4 inhibitor may increase serum levels of cinacalcet. Dose adjustment and monitoring of iPTH serum phosphorus and serum calcium may be required. (7.1)
- Cinacalcet is a strong inhibitor of CYP2D6. Dose adjustments may be required for concomitant medications that are predominantly metabolized by CYP2D6. (7.2)
USE IN SPECIFIC POPULATIONS
- Pediatric Use: A fatal outcome was reported in a pediatric clinical trial patient with severe hypocalcemia. Sensipar is not indicated for use in pediatric patients. (8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2019
- Secondary Hyperparathyroidism (HPT) in adult patients with chronic kidney disease (CKD) on dialysis. (1.1)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Secondary Hyperparathyroidism
1.2 Parathyroid Carcinoma
1.3 Primary Hyperparathyroidism
2 DOSAGE AND ADMINISTRATION
2.1 Administration
2.2 Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease on Dialysis
2.3 Patients with Parathyroid Carcinoma and Primary Hyperparathyroidism
2.4 Switching from Parsabiv (etelcalcetide) to Sensipar
2.5 Monitoring for Hypocalcemia
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypocalcemia
5.2 Upper Gastrointestinal Bleeding
5.3 Hypotension, Worsening Heart Failure and/or Arrhythmias
5.4 Adynamic Bone Disease
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Strong CYP3A4 Inhibitors
7.2 CYP2D6 Substrates
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease on Dialysis
14.2 Parathyroid Carcinoma
14.3 Patients with Hypercalcemia Due to Primary Hyperparathyroidism
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Secondary Hyperparathyroidism
Sensipar is indicated for the treatment of secondary hyperparathyroidism (HPT) in adult patients with chronic kidney disease (CKD) on dialysis [see Clinical Studies (14.1)].
Limitations of Use:
Sensipar is not indicated for use in patients with CKD who are not on dialysis because of an increased risk of hypocalcemia [see Warnings and Precautions (5.1)].
1.2 Parathyroid Carcinoma
Sensipar is indicated for the treatment of hypercalcemia in adult patients with Parathyroid Carcinoma [see Clinical Studies (14.2)].
1.3 Primary Hyperparathyroidism
Sensipar is indicated for the treatment of hypercalcemia in adult patients with primary HPT for whom parathyroidectomy would be indicated on the basis of serum calcium levels, but who are unable to undergo parathyroidectomy [see Clinical Studies (14.3)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Administration
Sensipar should be taken with food or shortly after a meal.
Sensipar tablets are administered orally and should always be taken whole and not chewed, crushed, or divided.
2.2 Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease on Dialysis
The recommended starting oral dose of Sensipar is 30 mg once daily. Serum calcium and serum phosphorus should be measured within 1 week and intact parathyroid hormone (iPTH) should be measured 1 to 4 weeks after initiation or dose adjustment of Sensipar [see Dosage and Administration (2.3)]. Sensipar should be titrated no more frequently than every 2 to 4 weeks through sequential doses of 30, 60, 90, 120, and 180 mg once daily to target iPTH levels of 150 to 300 pg/mL. Serum iPTH levels should be assessed no earlier than 12 hours after dosing with Sensipar.
Sensipar can be used alone or in combination with vitamin D sterols and/or phosphate binders.
During dose titration, serum calcium levels should be monitored frequently and if levels decrease below the normal range, appropriate steps should be taken to increase serum calcium levels, such as by providing supplemental calcium, initiating or increasing the dose of calcium-based phosphate binder, initiating or increasing the dose of vitamin D sterols, or temporarily withholding treatment with Sensipar [see Dosage and Administration (2.4) and Warnings and Precautions (5.1)].
2.3 Patients with Parathyroid Carcinoma and Primary Hyperparathyroidism
The recommended starting oral dose of Sensipar is 30 mg twice daily.
The dose of Sensipar should be titrated every 2 to 4 weeks through sequential doses of 30 mg twice daily, 60 mg twice daily, and 90 mg twice daily, and 90 mg 3 or 4 times daily as necessary to normalize serum calcium levels. Serum calcium should be measured within 1 week after initiation or dose adjustment of Sensipar [see Dosage and Administration (2.4) and Warnings and Precautions (5.1)].
2.4 Switching from Parsabiv (etelcalcetide) to Sensipar
Discontinue etelcalcetide for at least 4 weeks prior to starting Sensipar. Ensure corrected serum calcium is at or above the lower limit of normal prior to Sensipar initiation [see Warnings and Precautions (5.1)]. Initiate Sensipar treatment at a starting dose of 30 mg once daily.
2.5 Monitoring for Hypocalcemia
Once the maintenance dose has been established, serum calcium should be measured approximately monthly for patients with secondary hyperparathyroidism with CKD on dialysis, and every 2 months for patients with parathyroid carcinoma or primary hyperparathyroidism [see Dosage and Administration (2.2, 2.3)].
For secondary hyperparathyroidism patients with CKD on dialysis, if serum calcium falls below 8.4 mg/dL but remains above 7.5 mg/dL, or if symptoms of hypocalcemia occur, calcium-containing phosphate binders and/or vitamin D sterols can be used to raise serum calcium. If serum calcium falls below 7.5 mg/dL, or if symptoms of hypocalcemia persist and the dose of vitamin D cannot be increased, withhold administration of Sensipar until serum calcium levels reach 8.0 mg/dL and/or symptoms of hypocalcemia have resolved. Treatment should be reinitiated using the next lowest dose of Sensipar [see Dosage and Administration (2.2)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Sensipar treatment initiation is contraindicated if serum calcium is less than the lower limit of the normal range [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypocalcemia
Sensipar lowers serum calcium and can lead to hypocalcemia [see Adverse Reactions (6.1)]. Significant lowering of serum calcium can cause paresthesias, myalgias, muscle spasms, tetany, seizures, QT interval prolongation and ventricular arrhythmia. Life threatening events and fatal outcomes associated with hypocalcemia have been reported in patients treated with Sensipar, including in pediatric patients. The safety and effectiveness of Sensipar have not been established in pediatric patients [see Pediatric Use (8.4)].
Sensipar is not indicated for patients with CKD not on dialysis [see Indications and Usage (1)]. In patients with secondary HPT and CKD not on dialysis, the long-term safety and efficacy of Sensipar have not been established. Clinical studies indicate that Sensipar-treated patients with CKD not on dialysis have an increased risk for hypocalcemia compared with Sensipar-treated patients with CKD on dialysis, which may be due to lower baseline calcium levels. In a phase 3 study of 32 weeks duration and including 404 patients with CKD not on dialysis (302 cinacalcet, 102 placebo), in which the median dose for cinacalcet was 60 mg per day at the completion of the study, 80% of Sensipar-treated patients experienced at least one serum calcium value < 8.4 mg/dL compared with 5% of patients receiving placebo.
QT Interval Prolongation and Ventricular Arrhythmia
Decreases in serum calcium can also prolong the QT interval, potentially resulting in ventricular arrhythmia. Cases of QT prolongation and ventricular arrhythmia have been reported in patients treated with Sensipar. Patients with congenital long QT syndrome, history of QT interval prolongation, family history of long QT syndrome or sudden cardiac death, and other conditions that predispose to QT interval prolongation and ventricular arrhythmia may be at increased risk for QT interval prolongation and ventricular arrhythmias if they develop hypocalcemia due to Sensipar. Closely monitor corrected serum calcium and QT interval in patients at risk receiving Sensipar.
Seizures
In clinical studies, seizures (primarily generalized or tonic-clonic) were observed in 1.4% (43/3049) of Sensipar-treated patients and 0.7% (5/687) of placebo-treated patients. While the basis for the reported difference in seizure rate is not clear, the threshold for seizures is lowered by significant reductions in serum calcium levels. Monitor serum calcium levels in patients with seizure disorders receiving Sensipar.
Concurrent Administration with Other Calcium-Lowering Drug Products
Concurrent administration of Sensipar with calcium-lowering drugs including other calcium-sensing receptor agonists could result in severe hypocalcemia. Closely monitor serum calcium in patients receiving Sensipar and concomitant therapies known to lower serum calcium levels.
Patient Education and Hypocalcemia Treatment
Educate patients on the symptoms of hypocalcemia and advise them to contact a healthcare provider if they occur. If corrected serum calcium falls below the lower limit of normal or symptoms of hypocalcemia develop, start or increase calcium supplementation (including calcium, calcium-containing phosphate binders, and/or vitamin D sterols or increases in dialysate calcium concentration). Sensipar dose reduction or discontinuation of Sensipar may be necessary [see Dosage and Administration (2.2)].
5.2 Upper Gastrointestinal Bleeding
Cases of gastrointestinal bleeding, mostly upper gastrointestinal bleeding, have occurred in patients using calcimimetics, including Sensipar, from postmarketing and clinical trial sources. The exact cause of GI bleeding in these patients is unknown.
Patients with risk factors for upper GI bleeding (such as known gastritis, esophagitis, ulcers or severe vomiting) may be at increased risk for GI bleeding when receiving Sensipar treatment. Monitor patients for worsening of common GI adverse reactions of nausea and vomiting associated with Sensipar [see Adverse Reactions (6.1)] and for signs and symptoms of GI bleeding and ulcerations during Sensipar therapy. Promptly evaluate and treat any suspected GI bleeding.
5.3 Hypotension, Worsening Heart Failure and/or Arrhythmias
In postmarketing safety surveillance, isolated, idiosyncratic cases of hypotension, worsening heart failure, and/or arrhythmia have been reported in patients with impaired cardiac function, in which a causal relationship to Sensipar could not be completely excluded and which may be mediated by reductions in serum calcium levels [see Adverse Reactions (6.2)].
5.4 Adynamic Bone Disease
Adynamic bone disease may develop if iPTH levels are suppressed below 100 pg/mL. One clinical study evaluated bone histomorphometry in patients treated with Sensipar for 1 year. Three patients with mild hyperparathyroid bone disease at the beginning of the study developed adynamic bone disease during treatment with Sensipar. Two of these patients had iPTH levels below 100 pg/mL at multiple time points during the study. In three 6-month, phase 3 studies conducted in patients with CKD on dialysis, 11% of patients treated with Sensipar had mean iPTH values below 100 pg/mL during the efficacy-assessment phase. If iPTH levels decrease below 150 pg/mL in patients treated with Sensipar, the dose of Sensipar and/or vitamin D sterols should be reduced or therapy discontinued.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of labeling:
- Hypocalcemia [see Warnings and Precautions (5.1)]
- Upper Gastrointestinal Bleeding [see Warnings and Precautions (5.2)]
- Hypotension, Worsening Heart Failure and/or Arrhythmias [see Warnings and Precautions (5.3)]
- Adynamic Bone Disease [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease on Dialysis
In three double-blind, placebo-controlled clinical trials, 1126 patients with CKD on dialysis received study drug (656 Sensipar, 470 placebo) for up to 6 months. The most frequently reported adverse reactions are listed in Table 1.
Seizures were observed in 1.4% (13/910) of Sensipar-treated patients and 0.7% (5/641) of placebo-treated patients across all completed placebo-controlled trials.
Table 1. Adverse Reactions with Frequency ≥ 5% in Patients on Dialysis in Short-Term Studies for up to 6 Months Placebo Sensipar (n = 470) (n = 656) Event*: (%) (%) Nausea 19 31 Vomiting 15 27 Diarrhea 20 21 Myalgia 14 15 Dizziness 8 10 Hypertension 5 7 Asthenia 4 7 Anorexia 4 6 Pain Chest, Non-Cardiac 4 6 Dialysis Access Site Infection 4 5 *Included are events that were reported at a greater incidence in the Sensipar group than in the placebo group. In a randomized, double-blind placebo-controlled study of 3883 patients with secondary HPT and CKD receiving dialysis in which patients were treated for up to 64 months (mean duration of treatment was 21 months in the Sensipar group), the most frequently reported adverse reactions (incidence of ≥ 5% in the Sensipar group and a difference ≥ 1% compared to placebo) are listed in Table 2.
Table 2. Frequency of Adverse Reactions in Dialysis Patients Treated for up to 64 Months in a Long-Term Study1 Placebo (n = 1923) Sensipar (n = 1938) 3699 subject-years 4044 subject-years Percent of subjects reporting 90.9 93.2 Adverse Reactions (%) Nausea 15.5 29.1 Vomiting 13.7 25.6 Diarrhea 18.7 20.5 Dyspnea 11.5 13.4 Cough 9.8 11.7 Hypotension 10.5 11.6 Headache 9.6 11.5 Hypocalcemia 1.4 11.2 Muscle spasms 9.2 11.1 Abdominal pain 9.6 10.9 Abdominal pain upper 6.3 8.2 Hyperkalemia 6.1 8.1 Upper respiratory tract infection 6.3 7.6 Dyspepsia 4.6 7.4 Dizziness 4.7 7.3 Decreased appetite 3.5 5.9 Asthenia 3.8 5.4 Constipation 3.8 5.0 1 Adverse reactions that occurred in ≥ 5% frequency in the Sensipar group and a difference ≥ 1% compared to the placebo group (Safety Analysis Set).
Crude incidence rate = 100 * Total number of subjects with event/ n
n = Number of subjects receiving at least one dose of study drug.Additional adverse reaction rates from the long-term, randomized, double-blind placebo-controlled study for Sensipar versus placebo are as follows: seizure (2.5%, 1.6%), rash (2.2%, 1.9%), hypersensitivity reactions (9.4%, 8.3%).
Patients with Parathyroid Carcinoma and Primary Hyperparathyroidism
The safety profile of Sensipar in these patient populations is generally consistent with that seen in patients with CKD on dialysis. Forty six patients were treated with Sensipar in a single-arm study, 29 with Parathyroid Carcinoma and 17 with intractable pHPT. Nine (20%) of the patients withdrew from the study due to adverse events. The most frequent adverse reactions and the most frequent cause of withdrawal in these patient populations were nausea and vomiting. Severe or prolonged cases of nausea and vomiting can lead to dehydration and worsening hypercalcemia so careful monitoring of electrolytes is recommended in patients with these symptoms.
Eight patients died during treatment with Sensipar in this study, 7 with Parathyroid Carcinoma (24%) and 1 (6%) with intractable pHPT. Causes of death were cardiovascular (5 patients), multi-organ failure (1 patient), gastrointestinal hemorrhage (1 patient) and metastatic carcinoma (1 patient). Adverse events of hypocalcemia were reported in three patients (7%).
Seizures were observed in 0.7% (1/140) of cinacalcet-treated patients and 0.0% (0/46) of placebo-treated patients in all clinical studies.
Table 3. Adverse Reactions with Frequency ≥ 10% in a Single-Arm, Open-Label Study in Patients with Primary Hyperparathyroidism or Parathyroid Carcinoma Sensipar Parathyroid
Carcinoma
(n = 29)Intractable
pHPT
(n = 17)Total
(n = 46)n (%) n (%) n (%) Number of Subjects Reporting Adverse 28 (97) 17 (100) 45 (98) Reactions Nausea 19 (66) 10 (59) 29 (63) Vomiting 15 (52) 6 (35) 21 (46) Paresthesia 4 (14) 5 (29) 9 (20) Fatigue 6 (21) 2 (12) 8 (17) Fracture 6 (21) 2 (12) 8 (17) Hypercalcemia 6 (21) 2 (12) 8 (17) Anorexia 6 (21) 1 (6) 7 (15) Asthenia 5 (17) 2 (12) 7 (15) Dehydration 7 (24) 0 (0) 7 (15) Anemia 5 (17) 1 (6) 6 (13) Arthralgia 5 (17) 1 (6) 6 (13) Constipation 3 (10) 3 (18) 6 (13) Depression 3 (10) 3 (18) 6 (13) Headache 6 (21) 0 (0) 6 (13) Infection Upper Respiratory 3 (10) 2 (12) 5 (11) Pain Limb 3 (10) 2 (12) 5 (11) n = Number of subjects receiving at least one dose of study drug.
pHPT = primary hyperparathyroidism.In a randomized double-blind, placebo-controlled study of 67 patients with primary hyperparathyroidism for whom parathyroidectomy would be indicated on the basis of serum calcium levels, but who are unable to undergo surgery, the most common adverse reactions are listed in Table 4.
Table 4. Adverse Reactions Occurring in ≥ 10% of Subjects in a Double-Blind, Placebo-Controlled Study in Patients with Primary Hyperparathyroidism
Adverse Reaction Placebo
(n = 34)
n (%)Cinacalcet
(n = 33)
n (%)Nausea 6 (18) 10 (30) Muscle spasms 0 (0) 6 (18) Headache 2 (6) 4 (12) Back pain 2 (6) 4 (12) n = Number of subjects receiving at least one dose of study drug Coded using MedDRA version 16.0. Hypocalcemia
In 26-week studies of patients with secondary HPT and CKD on dialysis 66% of patients receiving Sensipar compared with 25% of patients receiving placebo developed at least one serum calcium value less than 8.4 mg/dL, whereas, 29% of patients receiving Sensipar compared with 11% of patients receiving placebo developed at least one serum calcium value less than 7.5 mg/dL. Less than 1% of patients in each group permanently discontinued study drug due to hypocalcemia.
In a randomized, double-blind, placebo-controlled study in patients with secondary HPT and CKD receiving dialysis in which patients were treated for up to 64 months (mean duration of treatment was 21 months in the cinacalcet group), 75% of patients receiving Sensipar compared with 29% of patients receiving placebo developed at least one serum calcium value less than 8.4 mg/dL and 33% of cinacalcet patients compared with 12% of patients receiving placebo had at least one serum calcium value less than 7.5 mg/dL. Most of the cases of severe hypocalcemia less than 7.5 mg/dL (21/33 = 64%) occurred during the first 6 months. In this trial, 1.1% of patients receiving Sensipar and 0.1% of patients receiving placebo permanently discontinued study drug due to hypocalcemia.
During a placebo-controlled part of a 52-week study in patients with primary HPT who met criteria for parathyroidectomy on the basis of corrected total serum calcium (> 11.3 mg/dL [2.82 mmol/L] and ≤ 12.5 mg/dL [3.12 mmol/L]), serum calcium less than 8.4 mg/dL was observed in 6.1% (2/33) of Sensipar-treated patients and 0% (0/34) of placebo-treated patients.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of Sensipar. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Rash and hypersensitivity reactions (including angioedema and urticaria), and myalgia
- Isolated, idiosyncratic cases of hypotension, worsening heart failure, and/or arrhythmia have been reported in patients with impaired cardiac function
- Gastrointestinal bleeding
- Chondrocalcinosis pyrophosphate (acute pseudogout)
- Hypocalcemia [see Warnings and Precautions (5.1)]
-
7 DRUG INTERACTIONS
7.1 Strong CYP3A4 Inhibitors
Cinacalcet is partially metabolized by CYP3A4. Dose adjustment of Sensipar may be required if a patient initiates or discontinues therapy with a strong CYP3A4 inhibitor (e.g., ketoconazole, itraconazole). The iPTH and serum calcium concentrations should be closely monitored in these patients [see Clinical Pharmacology (12.3)].
7.2 CYP2D6 Substrates
Cinacalcet is a strong inhibitor of CYP2D6. Dose adjustments may be required for concomitant medications that are predominantly metabolized by CYP2D6 (e.g., desipramine, metoprolol, and carvedilol) and particularly those with a narrow therapeutic index (e.g., flecainide and most tricyclic antidepressants) [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited case reports of Sensipar use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes. In animal reproduction studies, when female rats were exposed to cinacalcet during the period of organogenesis through to weaning at 2-3 times the systemic drug levels (based on AUC) at the maximum recommended human dose (MRHD) of 180 mg/day, peripartum and early postnatal pup loss and reduced pup body weight gain were observed in the presence of maternal hypocalcemia [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In pregnant female rats given oral gavage doses of 2, 25, 50 mg/kg/day cinacalcet during gestation, no teratogenicity was observed at doses up to 50 mg/kg/day (exposure 4 times those resulting with a human oral dose of 180 mg/day based on AUC comparison). Decreased fetal body weights were observed at all doses (less than 1 to 4 times a human oral dose of 180 mg/day based on AUC comparison) in conjunction with maternal toxicity (decreased food consumption and body weight gain).
In pregnant female rabbits given oral gavage doses of 2, 12, 25 mg/kg/day cinacalcet during gestation, no adverse fetal effects were observed (exposures less than with a human oral dose of 180 mg/day based on AUC comparisons). Reductions in maternal food consumption and body weight gain were seen at doses of 12 and 25 mg/kg/day. Cinacalcet has been shown to cross the placental barrier in rabbits.
In pregnant rats given oral gavage doses of 5, 15, 25 mg/kg/day cinacalcet during gestation through lactation, no adverse fetal or pup (post-weaning) effects were observed at 5 mg/kg/day (exposures less than with a human therapeutic dose of 180 mg/day based on AUC comparisons). Higher doses of 15 and 25 mg/kg/day cinacalcet (exposures 2 to 3 times a human oral dose of 180 mg/day based on AUC comparisons) were accompanied by maternal signs of hypocalcemia (periparturient mortality and early postnatal pup loss), and reductions in postnatal maternal and pup body weight gain.
8.2 Lactation
Risk Summary
There are no data regarding the presence of Sensipar in human milk or effects on the breastfed infant or on milk production. Studies in rats showed that cinacalcet was excreted in the milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Sensipar and any potential adverse effects on the breastfed infant from Sensipar or from the underlying maternal condition.
8.4 Pediatric Use
The safety and efficacy of Sensipar have not been established in pediatric patients.
The use of Sensipar for the treatment of secondary HPT in pediatric patients with CKD on dialysis was evaluated in two randomized, controlled studies (Pediatric Study 1 and Study 2) where 47 pediatric patients aged 6 years to less than 18 years received at least one dose of Sensipar and in one single-arm study (Pediatric Study 3) where 17 pediatric patients aged 28 days to less than 6 years received at least one dose of Sensipar. Dosing with Sensipar in Pediatric Study 1 was stopped because of a fatality in a Sensipar-treated individual. The individual was noted to be severely hypocalcemic at the time of death. The cause of death was multifactorial and a contribution of Sensipar to the death could not be excluded [see Warnings and Precautions (5.1)]. Study 1 was terminated and changes to Sensipar dosing after the fatality were implemented in Pediatric Study 2 and Study 3 to minimize the risk of severe hypocalcemia. The data in Pediatric Studies 2 and 3 were insufficient to establish the safety and efficacy of Sensipar for the treatment of secondary HPT in pediatric patients with CKD on dialysis. In aggregate, the pediatric studies did not establish a safe and effective Sensipar dosing regimen for the pediatric population.
8.5 Geriatric Use
Of the total number of subjects (n = 1136) in clinical studies of Sensipar, 26 percent were 65 and over, and 9 percent were 75 and over. No overall differences in the safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger subjects, but greater sensitivity of some older individuals cannot be ruled out [see Clinical Studies (14) and Clinical Pharmacology (12.3)].
8.6 Renal Impairment
No dosage adjustment is necessary for renal impairment [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Patients with moderate and severe hepatic impairment should have serum calcium, serum phosphorus, and iPTH levels monitored closely throughout treatment with Sensipar because cinacalcet exposure (AUC0-infinite) is increased by 2.4 and 4.2 fold, respectively, in these patients [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Overdosage of Sensipar may lead to hypocalcemia. In the event of overdosage, patients should be monitored for signs and symptoms of hypocalcemia and appropriate measures taken to correct serum calcium levels [see Warnings and Precautions (5.1)].
Since Sensipar is highly protein bound, hemodialysis is not an effective treatment for overdosage of Sensipar.
-
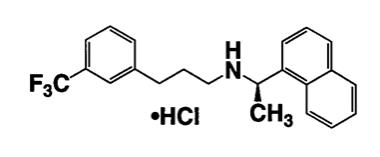
11 DESCRIPTION
Sensipar tablets contain the hydrochloride salt of the active ingredient cinacalcet, a positive modulator of the calcium sensing receptor . The empirical formula for cinacalcet is C22H22F3N•HCl with a molecular weight of 393.9 g/mol (hydrochloride salt) and 357.4 g/mol (free base). It has one chiral center having an R-absolute configuration. The R-enantiomer is the more potent enantiomer and has been shown to be responsible for pharmacodynamic activity. The hydrochloride salt of cinacalcet is a white to off-white, crystalline solid that is soluble in methanol or 95% ethanol and slightly soluble in water. The hydrochloride salt of cinacalcet is described chemically as N-[1-(R)-(-)-(1-naphthyl)ethyl]-3-[3-(trifluoromethyl)phenyl]-1-aminopropane hydrochloride and has the following structural formula:
Sensipar tablets are formulated as light-green, film-coated, oval-shaped tablets for oral administration in strengths of 30 mg, 60 mg, and 90 mg of cinacalcet as the free base equivalent (33 mg, 66 mg, and 99 mg as the hydrochloride salt, respectively).
Inactive Ingredients
The following are the inactive ingredients in Sensipar tablets: pre-gelatinized starch, microcrystalline cellulose, povidone, crospovidone, colloidal silicon dioxide and magnesium stearate. Tablets are coated with color (Opadry® II green), clear film coat (Opadry® clear), and carnauba wax.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The calcium-sensing receptor on the surface of the chief cell of the parathyroid gland is the principal regulator of PTH synthesis and secretion. Cinacalcet, the active ingredient in Sensipar, is a calcimimetic agent that directly lowers PTH levels by increasing the sensitivity of the calcium-sensing receptor to activation by extracellular calcium. The reduction in PTH is associated with a concomitant decrease in serum calcium levels.
12.2 Pharmacodynamics
Reduction in iPTH levels correlated with the plasma cinacalcet concentrations in patients with CKD. The nadir in iPTH level occurs approximately 2 to 6 hours post dose, corresponding with the maximum plasma concentration (Cmax) of cinacalcet. After steady-state cinacalcet concentrations are reached (which occurs within 7 days of dose change), serum calcium concentrations remain constant over the dosing interval in patients with CKD.
Reductions in PTH are associated with a decrease in bone turnover and bone fibrosis in patients with CKD on dialysis and uncontrolled secondary HPT.
12.3 Pharmacokinetics
Absorption and Distribution
After oral administration of cinacalcet, Cmax is achieved in approximately 2 to 6 hours. Cinacalcet Cmax and AUC(0-infinite) were increased by 82% and 68%, respectively, following administration with a high-fat meal compared with fasting in healthy volunteers. The Cmax and AUC(0-infinite) of cinacalcet were increased by 65% and 50%, respectively, when cinacalcet was administered with a low-fat meal compared with fasting.
After absorption, cinacalcet concentrations decline in a biphasic fashion with an initial half-life of approximately 6 hours and terminal half-life of 30 to 40 hours. Steady-state drug levels are achieved within 7 days, and the mean accumulation ratio is approximately 2 with once daily oral administration. The median accumulation ratio is approximately 2 to 5 with twice daily oral administration. The AUC and Cmax of cinacalcet increase proportionally over the dose range of 30 to 180 mg once daily. The pharmacokinetic profile of cinacalcet does not change over time with once daily dosing of 30 to 180 mg. The volume of distribution is approximately 1000 L, indicating extensive distribution. Cinacalcet is approximately 93% to 97% bound to plasma protein(s). The ratio of blood cinacalcet concentration to plasma cinacalcet concentration is 0.80 at a blood cinacalcet concentration of 10 ng/mL.
Metabolism and Excretion
Cinacalcet is metabolized by multiple enzymes, primarily CYP3A4, CYP2D6, and CYP1A2. After administration of a 75 mg radiolabeled dose to healthy volunteers, cinacalcet was metabolized via: 1) oxidative N-dealkylation to hydrocinnamic acid and hydroxy-hydrocinnamic acid, which are further metabolized via β-oxidation and glycine conjugation; the oxidative N-dealkylation process also generates metabolites that contain the naphthalene ring; and 2) oxidation of the naphthalene ring on the parent drug forming dihydrodiols, which are further conjugated with glucuronic acid. The plasma concentrations of the major circulating metabolites, including the cinnamic acid derivatives and glucuronidated dihydrodiols, markedly exceed the parent drug concentrations. The hydrocinnamic acid metabolite and glucuronide conjugates have minimal or no calcimimetic activity. Renal excretion of metabolites was the primary route of elimination of radioactivity. Approximately 80% of the dose was recovered in the urine and 15% in the feces.
Specific Populations
Age: Geriatric Population
The pharmacokinetic profile of cinacalcet in geriatric patients (age ≥ 65 years, n = 12) is similar to that for patients who are < 65 years of age (n = 268) [see Use in Specific Populations (8.5)].
Hepatic Impairment
The disposition of a 50 mg Sensipar single dose was compared between patients with hepatic impairment and patients with normal hepatic function. Cinacalcet exposure (AUC(0-infinite)) was comparable between healthy volunteers and patients with mild hepatic impairment. However, in patients with moderate and severe hepatic impairment (as indicated by the Child-Pugh method), cinacalcet exposures (AUC(0-infinite)) were 2.4 and 4.2 fold higher, respectively, than that in healthy volunteers. The mean half-life of cinacalcet increased from 49 hours in healthy volunteers to 65 hours and 84 hours in patients with moderate and severe hepatic impairment, respectively. Protein binding of cinacalcet is not affected by impaired hepatic function [see Use in Specific Populations (8.7)].
Renal Impairment
The pharmacokinetic profile of a 75 mg Sensipar single dose in patients with mild, moderate, and severe renal impairment, and those on hemodialysis or peritoneal dialysis is comparable with that in healthy volunteers [see Use in Specific Populations (8.6)].
Drug Interactions
In vitro studies indicate that cinacalcet is a strong inhibitor of CYP2D6, but not an inhibitor of CYP1A2, CYP2C9, CYP2C19, and CYP3A4. In vitro induction studies indicate that cinacalcet is not an inducer of CYP450 enzymes. Tables 5 and 6 list the findings from in vivo drug-drug interaction studies.
Table 5. Effect of co-administered drugs on cinacalcet Co-administered drug and dosing
regimenCinacalcet Dose* Mean change in
AUC(0-inf)Mean change in
Cmax200 mg ketoconazole twice daily for
7 days90 mg on day 5 ↑127% ↑116% 1500 mg calcium carbonate, single dose 100 mg ↓6% ↓5% 80 mg pantoprazole daily for 3 days 90 mg on day 3 ↑1% ↓3% 2400 mg sevelamer HCl three times a
day for 2 days90 mg on day 1 with first
dose of sevelamer↓4% ↓7% *Single dose. Table 6. Effect of cinacalcet co-administration on other drugs Cinacalcet dosing
regimenCo-administered drug Name and Dose Mean change in AUC(0-inf) Mean change in Cmax 30 mg twice daily for
8 days25 mg warfarin*
tablet†↑1% for R-warfarin
↓1% for S-warfarin↓10% for R-warfarin
↓12% for S-warfarin90 mg daily for 7 days to CYP2D6 extensive
metabolizers50 mg desipramine† ↑264% ↑75% 90 mg daily for 5 days 2 mg midazolam† ↑5% ↓5% 25 or 100 mg single dose
to CYP2D6 extensive
metabolizers50 mg amitriptyline
single dose↑21-22% for amitriptyline
↑17-23% for nortriptyline‡↑13-21% for amitriptyline
↑11-15% for nortriptyline‡*No significant change in prothrombin time.
†Single dose on day 5.
‡Nortriptyline is an active metabolite of amitriptyline. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Standard lifetime dietary carcinogenicity bioassays were conducted in mice and rats. Mice were given cinacalcet at dietary doses of 15, 50, and 125 mg/kg/day in males and 30, 70, and 200 mg/kg/day in females (exposures up to 2 times those resulting with a human oral dose of 180 mg/day based on AUC comparison). Rats were given dietary doses of 5, 15, and 35 mg/kg/day in males and 5, 20, and 35 mg/kg/day in females (exposures up to 2 times those resulting with a human oral dose of 180 mg/day based on AUC comparison). No increased incidence of tumors was observed following treatment with cinacalcet.Mutagenicity
Cinacalcet was not genotoxic in the Ames bacterial mutagenicity assay, nor in the Chinese Hamster Ovary (CHO) cell HGPRT forward mutation assay and CHO cell chromosomal aberration assay, with and without metabolic activation, nor in the in vivo mouse micronucleus assay.Impairment of Fertility
Female rats were given oral gavage doses of 5, 25, and 75 mg/kg/day cinacalcet beginning 2 weeks before mating and continuing through gestation day 7. Male rats were given oral doses 4 weeks prior to mating, during mating (3 weeks) and 2 weeks postmating. No effects were observed in male or female fertility at 5 and 25 mg/kg/day (exposures up to 3 times those resulting with a human oral dose of 180 mg/day based on AUC comparison). At 75 mg/kg/day, there were slight adverse effects (slight decreases in body weight and food consumption) in males and females. -
14 CLINICAL STUDIES
14.1 Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease on Dialysis
Three 6-month, multicenter, randomized, double-blind, placebo-controlled clinical studies of similar design were conducted in patients with CKD on dialysis. A total of 665 patients were randomized to Sensipar and 471 patients to placebo. The mean age of the patients was 54 years, 62% were male, and 52% were Caucasian. The average baseline iPTH level by the Nichols IRMA was 712 pg/mL, with 26% of the patients having a baseline iPTH level > 800 pg/mL. The mean baseline Ca x P product was 61 mg2/dL2. The average duration of dialysis prior to study enrollment was 67 months. Ninety-six percent of patients were on hemodialysis and 4% on peritoneal dialysis. At study entry, 66% of the patients were receiving vitamin D sterols and 93% were receiving phosphate binders. Sensipar (or placebo) was initiated at a dose of 30 mg once daily and titrated every 3 or 4 weeks to a maximum dose of 180 mg once daily to achieve an iPTH of ≤ 250 pg/mL. The dose was not increased if a patient had any of the following: iPTH ≤ 200 pg/mL, serum calcium < 7.8 mg/dL, or any symptoms of hypocalcemia. If a patient experienced symptoms of hypocalcemia or had a serum calcium < 8.4 mg/dL, calcium supplements and/or calcium-based phosphate binders could be increased. If these measures were insufficient, the vitamin D dose could be increased. Approximately 70% of patients in the Sensipar arm and 80% of the patients in the placebo arm completed the 6-month studies. In the primary efficacy analysis, 40% of the patients on Sensipar and 5% of placebo-treated patients achieved an iPTH ≤ 250 pg/mL (p < 0.001) (Table 7, Figure 1). These studies showed that Sensipar reduced iPTH while lowering Ca x P, calcium, and phosphorus levels (Table 7, Figure 2). The median dose of Sensipar at the completion of the studies was 90 mg. Patients with milder disease typically required lower doses.
Similar results were observed when either the iPTH or biointact PTH (biPTH) assay was used to measure PTH levels in CKD patients on dialysis; treatment with cinacalcet did not alter the relationship between iPTH and biPTH.
Table 7. Effects of Sensipar on iPTH, Ca x P, Serum Calcium, and Serum Phosphorus in 6-month Phase 3 Studies (Patients on Dialysis) Study 1 Study 2 Study 3 Placebo Sensipar Placebo Sensipar Placebo Sensipar (n = 205) (n = 205) (n = 165) (n = 166) (n = 101) (n = 294) iPTH Baseline (pg/mL): Median
Mean (SD)535
651 (398)537
636 (341)556
630 (317)547
652 (372)670
832 (486)703
848 (685)Evaluation Phase (pg/mL) 563 275 592 238 737 339 Median Percent Change +3.8 -48.3 +8.4 -54.1 +2.3 -48.2 Patients Achieving Primary Endpoint (iPTH ≤ 250 pg/mL) (%)a 4% 41%** 7% 46%** 6% 35%** Patients Achieving ≥ 30% Reduction in iPTH (%)a 11% 61% 12% 68% 10% 59% Patients Achieving iPTH ≤ 250 pg/mL and Ca x P < 55 mg2/dL2 (%) 1% 32% 5% 35% 5% 28% Ca x P Baseline (mg2/dL2) 62 61 61 61 61 59 Evaluation Phase (mg2/dL2) 59 52 59 47 57 48 Median Percent Change -2.0 -14.9 -3.1 -19.7 -4.8 -15.7 Calcium Baseline (mg/dL) 9.8 9.8 9.9 10.0 9.9 9.8 Evaluation Phase (mg/dL) 9.9 9.1 9.9 9.1 10.0 9.1 Median Percent Change +0.5 -5.5 +0.1 -7.4 +0.3 -6.0 Phosphorus Baseline (mg/dL) 6.3 6.1 6.1 6.0 6.1 6.0 Evaluation Phase (mg/dL) 6.0 5.6 5.9 5.1 5.6 5.3 Median Percent Change -1.0 -9.0 -2.4 -12.4 -5.6 -8.6 ** p < 0.001 compared with placebo; p-values presented for primary endpoint only.
a iPTH value based on averaging over the evaluation phase (defined as weeks 13 to 26 in studies 1 and 2 and weeks 17 to 26 in study 3).
Values shown are medians unless indicated otherwise.Figure 1. Mean (SE) iPTH Values (Pooled Phase 3 Studies)
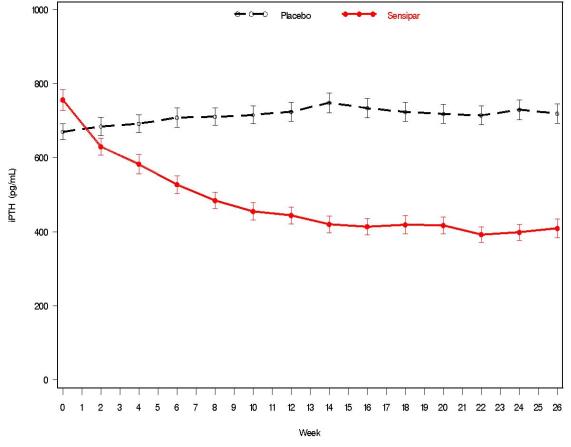
Data are presented for patients who completed the studies; Placebo (n = 342), Sensipar (n = 439). Figure 2. Mean (SE) Ca x P Values (Pooled Phase 3 Studies)
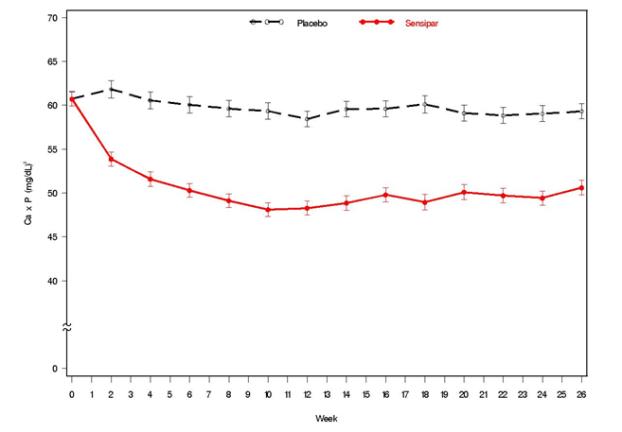
Data are presented for patients who completed the studies; Placebo (n = 342), Sensipar (n = 439). Reductions in iPTH and Ca x P were maintained for up to 12 months of treatment.
Sensipar decreased iPTH and Ca x P levels regardless of disease severity (i.e., baseline iPTH value), duration of dialysis, and whether or not vitamin D sterols were administered. Approximately 60% of patients with mild (iPTH ≥ 300 to ≤ 500 pg/mL), 41% with moderate (iPTH > 500 to 800 pg/mL), and 11% with severe (iPTH > 800 pg/mL) secondary HPT achieved a mean iPTH value of ≤ 250 pg/mL. Plasma iPTH levels were measured using the Nichols IRMA.
14.2 Parathyroid Carcinoma
Twenty-nine patients with Parathyroid Carcinoma were enrolled in a single-arm, open-label study. The study consisted of two phases, a dose-titration phase and a maintenance phase. Patients initially received 30 mg cinacalcet twice daily and then were titrated every 2 weeks to a maximum dose of 90 mg four times daily. Dosage escalation during the variable-length (2 to 16 weeks) titration phase continued until the serum calcium concentration was ≤ 10 mg/dL (2.5 mmol/L), the patient reached the highest possible dosage, or adverse events precluded further dosage increases.
Twenty-nine patients entered the study. The median exposure to cinacalcet was 229 days (range: 1 to 1051). At baseline the mean (SE) serum calcium was 14.1 (0.4) mg/dL. At the end of the titration phase, the mean (SE) serum calcium was 12.4 (0.5) mg/dL, which is a mean reduction of 1.7 (0.6) mg/dL from baseline. Figure 3 illustrates mean serum calcium (mg/dL) over time for all patients still on study at each time point from the beginning of titration to study visit week 80. Daily dose during the study ranged from 30 mg twice daily to 90 mg four times daily.
Figure 3. Serum Calcium Values in Patients with Parathyroid Carcinoma Receiving Sensipar at Baseline, Titration, and Maintenance Phase

n = Number of patients with non-missing values at the timepoint.
End of Titration (EOT) phase could occur at any visit from week 2 to 16. Patients at EOT are those who completed titration.14.3 Patients with Hypercalcemia Due to Primary Hyperparathyroidism
Seventeen patients with severe hypercalcemia due to primary HPT, who had failed or had contraindications to parathyroidectomy, participated in an open-label, single-arm study. The study consisted of two phases, a dose-titration phase and a maintenance phase. In this trial, severe hypercalcemia was defined as a screening serum calcium level of > 12.5 mg/dL. Patients initially received 30 mg cinacalcet twice daily and then were titrated every 2 weeks to a maximum dose of 90 mg 4 times daily. Dosage escalation during the variable-length (2 to 16 weeks) titration phase continued until the serum calcium concentration was ≤ 10 mg/dL (2.5 mmol/L), the patient reached the highest possible dosage, or adverse events precluded further dosage increases.
Seventeen patients entered the study. The median exposure to cinacalcet was 270 days (range: 32 to 1,105). At baseline the mean (SE) serum calcium was 12.7 (0.2) mg/dL. At the end of the titration phase the mean (SE) serum calcium was 10.4 (0.3) mg/dL, which is a mean reduction of 2.3 (0.3) mg/dL from baseline. Figure 4 illustrates mean serum calcium (mg/dL) over time for all patients still on study at each time point from the beginning of titration to study visit week 80. Daily dose during the study ranged from 30 mg twice a day to 90 mg four times a day.
Figure 4. Mean Serum Calcium (SE) at Baseline, End of Titration, and Scheduled Maintenance Visits (Patients with Severe intractable primary HPT)
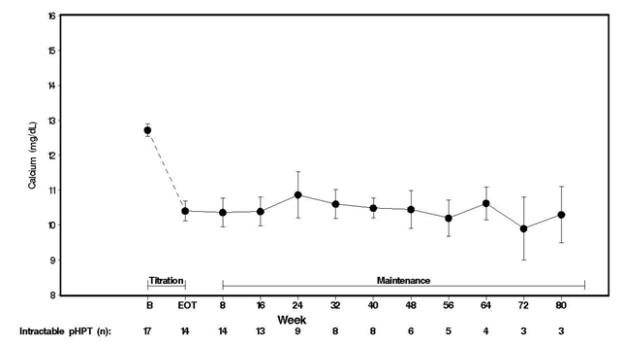
n = Number of patients with non-missing values at the timepoint.
End of Titration (EOT) phase could occur at any visit from week 2 to 16. Patients at EOT are those who completed titration.Sixty-seven patients with primary HPT who met criteria for parathyroidectomy on the basis of corrected total serum calcium (> 11.3 mg/dL [2.82 mmol/L] and ≤ 12.5 mg/dL [3.12 mmol/L]), but who were unable to undergo parathyroidectomy participated in a randomized, double-blind, placebo-controlled study. A total of 33 patients were randomized to Sensipar and 34 patients randomized to placebo. The mean age of the patients was 72 years, 52% were female, 61% were Caucasian, and 5% were Blacks. The study started with a 12-week titration phase, followed by a 16-week efficacy-assessment phase. Cinacalcet was initiated at a dose of 30 mg twice daily and titrated to maintain a corrected total serum calcium concentration within the normal range. During the efficacy period a significantly higher percentage of cinacalcet-treated patients compared with the placebo-treated patients achieved mean corrected total serum calcium concentration (≤ 10.3 mg/dL [2.57 mmol/L], 75.8% vs 0%, p < 0.001) and ≥ 1 mg/dL [0.25 mmol/L] decrease from baseline in mean corrected total serum calcium concentration (84.8% vs 5.9%, p < 0.001). The median dose of Sensipar at the completion of the study was 60 mg/day.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Sensipar 30 mg tablets are formulated as light-green, film-coated, oval-shaped tablets marked with “AMG” on one side and “30” on the opposite side, packaged in bottles of 30 tablets. (NDC 55513-073-30)
Sensipar 60 mg tablets are formulated as light-green, film-coated, oval-shaped tablets marked with “AMG” on one side and “60” on the opposite side, packaged in bottles of 30 tablets. (NDC 55513-074-30)
Sensipar 90 mg tablets are formulated as light-green, film-coated, oval-shaped tablets marked with “AMG” on one side and “90” on the opposite side, packaged in bottles of 30 tablets. (NDC 55513-075-30)
Storage
Store at 25°C (77°F); excursions permitted from 15°C to 30°C (59°F to 86°F). [See USP controlled room temperature].
-
17 PATIENT COUNSELING INFORMATION
-
Hypocalcemia: Advise patients to report symptoms of hypocalcemia, including paresthesias, myalgias, muscle spasms, and seizures, to their healthcare provider [see Warnings and Precautions (5.1)].
-
Upper Gastrointestinal Bleeding: Advise patients to report any symptoms of upper gastrointestinal bleeding to their health care provider [see Warnings and Precautions (5.2)].
-
Heart Failure: Advise patients with heart failure that use of Sensipar may worsen their heart failure and additional monitoring may be required [see Warnings and Precautions (5.3)].
- Advise patients to report nausea and vomiting to their health care provider [see Adverse Reactions (6.1)].
- Advise patients to take Sensipar with food or shortly after a meal and to take the tablets whole and not divide them [see Dosage and Administration (2.1)].
- Inform patients of the importance of regular blood tests, in order to monitor the safety and efficacy of Sensipar therapy.
Sensipar® (cinacalcet) Tablets
Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, California 91320-1799Patent: http://pat.amgen.com/sensipar/
© 2004-2019 Amgen Inc. All rights reserved.
www.sensipar.com
1-800-77-AMGEN (1-800-772-6436)
1xxxxxx – v14
-
Hypocalcemia: Advise patients to report symptoms of hypocalcemia, including paresthesias, myalgias, muscle spasms, and seizures, to their healthcare provider [see Warnings and Precautions (5.1)].
-
PRINCIPAL DISPLAY PANEL
AMGEN®
NDC 55513-073-30
Sensipar®
(cinacalcet) Tablets
Rx Only
30 tablets
30 mg
Each tablet contains:
Cinacalcet 30 mg (equivalent to 33 mg of cinacalcet hydrochloride).
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
See USP controlled room temperature.
Dispense in tight, light-resistant container per USP.
Dosage : See Package Insert.
© 2004-2018 Amgen Inc. All rights reserved.
Patent : http://pat.amgen.com/sensipar/
Distributed by : Amgen,
One Amgen Center Drive,
Thousand Oaks, CA 91320-1799
Made in Japan

-
PRINCIPAL DISPLAY PANEL
AMGEN®
NDC 55513-074-30
Sensipar®
(cinacalcet) Tablets
Rx Only
30 tablets
60 mg
Each tablet contains:
Cinacalcet 60 mg (equivalent to 66 mg of cinacalcet hydrochloride).
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
See USP controlled room temperature.
Dispense in tight, light-resistant container per USP.
Dosage : See Package Insert.
© 2004-2018 Amgen Inc. All rights reserved.
Patent : http://pat.amgen.com/sensipar/
Distributed by : Amgen,
One Amgen Center Drive,
Thousand Oaks, CA 91320-1799
Made in Japan

-
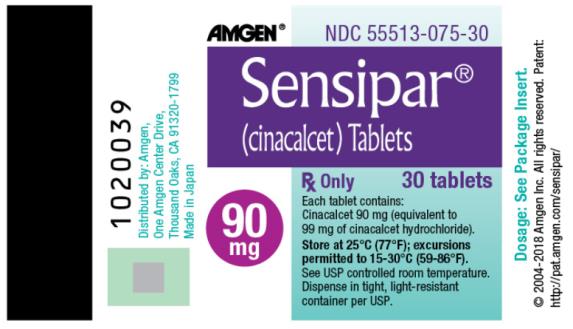
PRINCIPAL DISPLAY PANEL
AMGEN®
NDC 55513-075-30
Sensipar®
(cinacalcet) Tablets
Rx Only
30 tablets
90 mg
Each tablet contains:
Cinacalcet 90 mg (equivalent to 99 mg of cinacalcet hydrochloride).
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
See USP controlled room temperature.
Dispense in tight, light-resistant container per USP.
Dosage : See Package Insert.
© 2004-2018 Amgen Inc. All rights reserved.
Patent : http://pat.amgen.com/sensipar/
Distributed by : Amgen,
One Amgen Center Drive,
Thousand Oaks, CA 91320-1799
Made in Japan
-
INGREDIENTS AND APPEARANCE
SENSIPAR
cinacalcet hydrochloride tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55513-073 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CINACALCET HYDROCHLORIDE (UNII: 1K860WSG25) (CINACALCET - UNII:UAZ6V7728S) CINACALCET 30 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color GREEN (light green) Score no score Shape OVAL Size 10mm Flavor Imprint Code AMG;30 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55513-073-30 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/04/2004 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021688 04/04/2004 SENSIPAR
cinacalcet hydrochloride tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55513-074 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CINACALCET HYDROCHLORIDE (UNII: 1K860WSG25) (CINACALCET - UNII:UAZ6V7728S) CINACALCET 60 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color green (light green) Score no score Shape OVAL Size 12mm Flavor Imprint Code AMG;60 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55513-074-30 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/04/2004 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021688 04/04/2004 SENSIPAR
cinacalcet hydrochloride tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55513-075 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CINACALCET HYDROCHLORIDE (UNII: 1K860WSG25) (CINACALCET - UNII:UAZ6V7728S) CINACALCET 90 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color green (light green) Score no score Shape OVAL Size 14mm Flavor Imprint Code AMG;90 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55513-075-30 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/04/2004 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021688 04/04/2004 Labeler - Amgen Inc (039976196) Establishment Name Address ID/FEI Business Operations Patheon Inc. 240769596 ANALYSIS(55513-073, 55513-074, 55513-075) , LABEL(55513-073, 55513-074, 55513-075) , MANUFACTURE(55513-073, 55513-074, 55513-075) , PACK(55513-073, 55513-074, 55513-075)