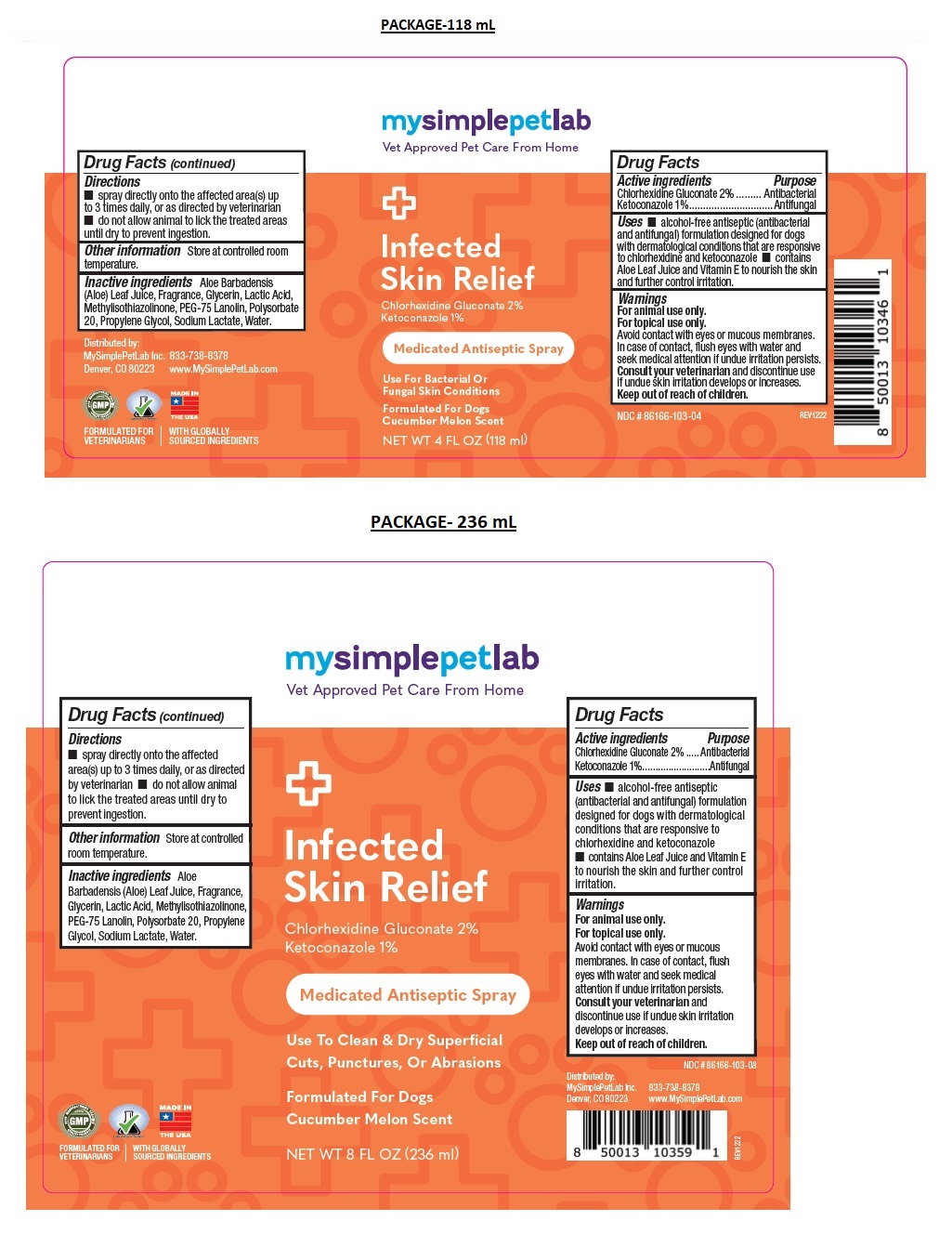
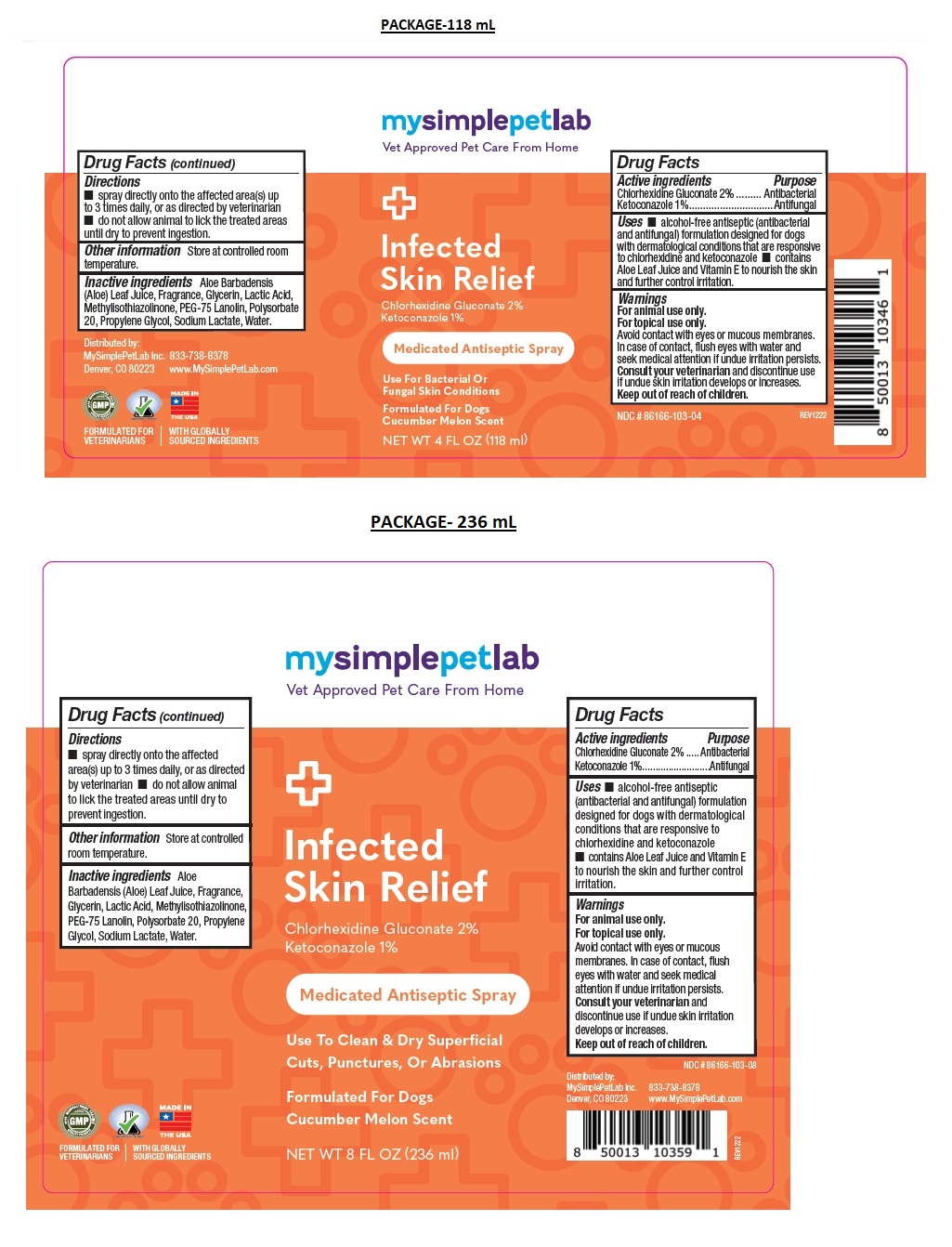
Label: MYSIMPLEPETLAB INFECTED SKIN RELIEF- chlorhexidine gluconate, ketoconazole spray
- NDC Code(s): 86166-103-04, 86166-103-08
- Packager: Mysimplepetlab Inc
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purpose
- Uses
-
Warnings
For animal use only.
For topical use only.
Avoid contact with eyes or mucous membranes. In case of contact, flush eyes with water and seek medical attention if undue irritation persists.
Consult your veterinarian and discontinue use if undue skin irritation develops or increases.
Keep out of the reach of children. - Directions
- Other information
- Inactive ingredients
-
SPL UNCLASSIFIED SECTION
Vet Approved Pet Care From Home
Medicated Antiseptic Spray
Use For Bacterial Or Fungal Skin Conditions
Formulated For Dogs
Cucumber Melon ScentFORMULATED FOR VETERINARIANS l WITH GLOBALLY SOURCED INGREDIENTS
MADE IN THE USA
Distributed by:
MySimplePetLab Inc.
Denver, CO 80223833-738-8378
www.MySimplePetLab.com - Packaging
-
INGREDIENTS AND APPEARANCE
MYSIMPLEPETLAB INFECTED SKIN RELIEF
chlorhexidine gluconate, ketoconazole sprayProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:86166-103 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 2 g in 100 mL KETOCONAZOLE (UNII: R9400W927I) (KETOCONAZOLE - UNII:R9400W927I) KETOCONAZOLE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) PEG-75 LANOLIN (UNII: 09179OX7TB) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM LACTATE (UNII: TU7HW0W0QT) WATER (UNII: 059QF0KO0R) Product Characteristics Color orange (Pink) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86166-103-04 118 mL in 1 BOTTLE, SPRAY 2 NDC:86166-103-08 236 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/01/2023 Labeler - Mysimplepetlab Inc (118813078)