Label: METHENAMINE HIPPURATE tablet
- NDC Code(s): 50742-142-01
- Packager: Ingenus Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of methenamine hippurate tablets and other antibacterial drugs, methenamine hippurate tablets should be used ...
-
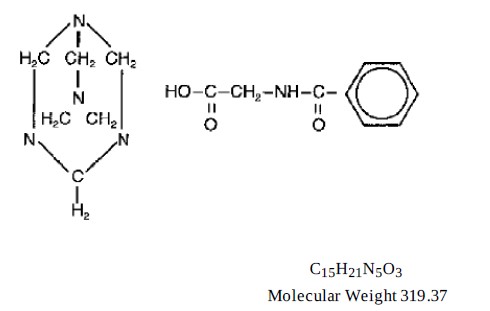
DESCRIPTIONMethenamine Hippurate Tablets, USP is a urinary tract antiseptic drug. Each peach color, capsule-shaped bi-convex tablet contains 1 g methenamine hippurate which is the hippuric acid salt of ...
-
ACTIONSMicrobiology - Methenamine hippurate tablets have antibacterial activity because the methenamine component is hydrolyzed to formaldehyde in acid urine. Hippuric acid, the other component, has ...
-
INDICATIONSMethenamine hippurate tablets are indicated for prophylactic or suppressive treatment of frequently recurring urinary tract infections when long-term therapy is considered necessary. This drug ...
-
CONTRAINDICATIONSMethenamine hippurate tablets are contraindicated in patients with renal insufficiency, severe hepatic insufficiency, or severe dehydration. Methenamine preparations should not be given to ...
-
WARNINGSLarge doses of methenamine (8 grams daily for 3 to 4 weeks) have caused bladder irritation, painful and frequent micturition, albuminuria, and gross hematuria.
-
PRECAUTIONSPrescribing methenamine hippurate tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and ...
-
Geriatric UseClinical studies of methenamine hippurate tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other ...
-
Information for PatientsPatients should be counseled that antibacterial drugs including methenamine hippurate tablets should only be used to treat bacterial infections. They do not treat viral infections (e.g., the ...
-
ADVERSE REACTIONSMinor adverse reactions have been reported in less than 3.5% of patients treated. These reactions have included nausea, upset stomach, dysuria and rash. To report SUSPECTED ADVERSE REACTIONS ...
-
DOSAGE AND ADMINISTRATION1 tablet (1.0 g) twice daily (morning and night) for adults and pediatric patients over 12 years of age. 1/2 to 1 tablet (0.5 to 1.0 g) twice daily (morning and night) for pediatric patients 6 ...
-
HOW SUPPLIEDPeach colored, capsule shaped, biconvex tablets debossed with "I" and "7"on one side and scoreline on two sides, free from mottled surface in bottles of 100 (NDC 50742- 142-01). Store at 25°C ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELContainer Label - 100s Count
-
INGREDIENTS AND APPEARANCEProduct Information