Label: TOP VALUE EFFERVESCENT COLD RELIEF- aspirin, chlorpheniramine maleate, phenylephrine bitartrate tablet, effervescent
- NDC Code(s): 50201-7682-2
- Packager: Tower Laboratories Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Purpose
- Active ingredients
- Uses
-
Warnings
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include: ■ hives ■ facial swelling ■ asthma (wheezing) ■ shock
Stomach bleeding warning: This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause severe stomach bleeding. The chance is higher if you
■ are age 60 or older ■ have had stomach ulcers or bleeding problems ■ take a blood thinning (anticoagulant) or steroid drug ■ take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others) ■ have 3 or more alcoholic drinks every day while using this product ■ take more or for a longer time than directed
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
-
Do not use
■ if you have ever had an allergic reaction to any other pain reliever/fever reducer
■ if you are allergic to aspirin
■ if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
-
Ask a doctor before use if
■ stomach bleeding warning applies to you ■ you have a history of stomach problems, such as heartburn ■ you have high blood pressure, heart disease, liver cirrhosis, or kidney disease ■ you are taking a diuretic ■ you are taking sedatives or tranquilizers
You have ■ glaucoma ■ diabetes ■ thyroid disease ■ trouble urinating due to an enlarged prostate gland ■ a breathing problem such as emphysema or chronic bronchitis ■ been placed on a sodium-restricted diet
- Ask a doctor or pharmacist before use if you are
-
When using this product
■ do not take more than 8 tablets (adults and children 12 years and over) in a 24-hour period or as directed by doctor.
■ do not use more than directed
■ you may get drowsy
■ avoid alcoholic drinks
■ excitability may occur, especially in children
■ alcohol, sedatives and tranquilizers may increase drowsiness
■ be careful when driving a motor vehicle or operating machinery
-
Stop use and ask a doctor if
■ you experience any of the following signs of stomach bleeding
■ feel faint ■ vomit blood ■ have bloody or black stools ■ have stomach pain that does not get better
■ an allergic reaction occurs. Seek medical help right away.
■ pain or nasal congestion gets worse or lasts more than 7 days
■ fever gets worse or lasts more than 3 days
■ new symptoms occur
■ redness or swelling is present
■ ringing in the ears or a loss of hearing occurs
■ nervousness, dizziness or sleeplessness occurs
- If pregnant or breast-feeding,
- In case of overdose,
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive Ingredients:
- QUESTIONS
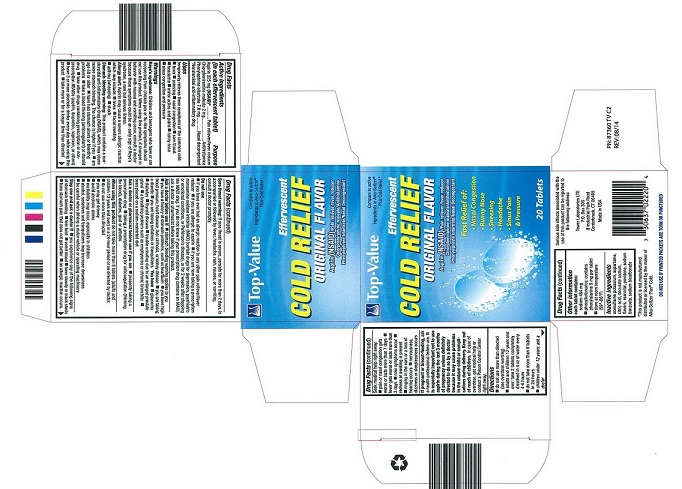
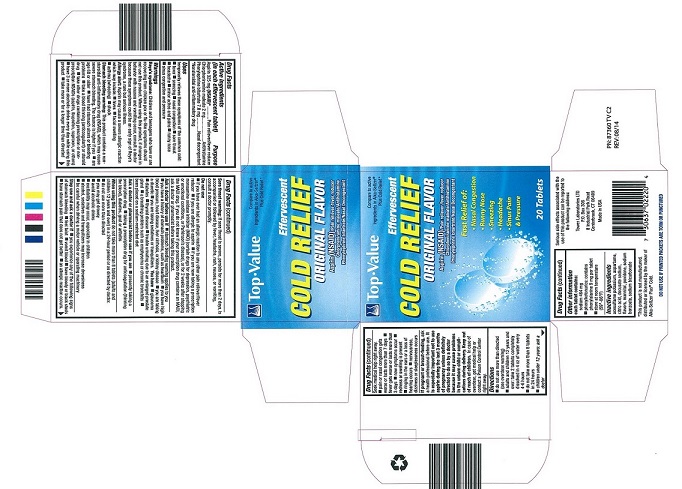
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TOP VALUE EFFERVESCENT COLD RELIEF
aspirin, chlorpheniramine maleate, phenylephrine bitartrate tablet, effervescentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50201-7682 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 325 mg PHENYLEPHRINE BITARTRATE (UNII: 27O3Q5ML57) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE BITARTRATE 7.8 mg CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 2 mg Inactive Ingredients Ingredient Name Strength ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) SODIUM BENZOATE (UNII: OJ245FE5EU) DOCUSATE SODIUM (UNII: F05Q2T2JA0) ASPARTAME (UNII: Z0H242BBR1) SODIUM BICARBONATE (UNII: 8MDF5V39QO) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) MANNITOL (UNII: 3OWL53L36A) POVIDONE K30 (UNII: U725QWY32X) Product Characteristics Color white Score no score Shape ROUND Size 25mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50201-7682-2 10 in 1 CARTON 07/01/2010 1 2 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 07/01/2010 Labeler - Tower Laboratories Ltd (001587203) Registrant - Tower Laboratories Ltd (001587203) Establishment Name Address ID/FEI Business Operations Tower Laboratories Ltd 869024500 manufacture(50201-7682) , label(50201-7682) , pack(50201-7682)