Label: ILUVIEN- fluocinolone acetonide implant
- NDC Code(s): 68611-190-02
- Packager: Alimera Sciences, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 21, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ILUVIEN® safely and effectively. See full prescribing information for ILUVIEN. ILUVIEN® (fluocinolone acetonide intravitreal ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Diabetic Macular Edema - ILUVIEN® is indicated for the treatment of diabetic macular edema (DME) in patients who have been previously treated with a course of corticosteroids and did not have ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Dosing Information - For ophthalmic intravitreal injection. The initial prescription and renewal of the medication order of ILUVIEN should be made by a physician only after ...
-
3 DOSAGE FORMS AND STRENGTHSILUVIEN is a non-bioerodable intravitreal implant in a drug delivery system containing 0.19 mg fluocinolone acetonide, designed to release fluocinolone acetonide at an initial rate of 0.25 mcg/day ...
-
4 CONTRAINDICATIONS4.1 Ocular or Periocular Infections - ILUVIEN is contraindicated in patients with active or suspected ocular or periocular infections including most viral disease of the cornea and conjunctiva ...
-
5 WARNINGS AND PRECAUTIONS5.1 Intravitreal Injection-related Effects - Intravitreal injections, including those with ILUVIEN, have been associated with endophthalmitis, eye inflammation, increased or decreased ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies of ILUVIEN use in pregnant women to inform a drug-associated risk. Animal reproduction studies have not been ...
-
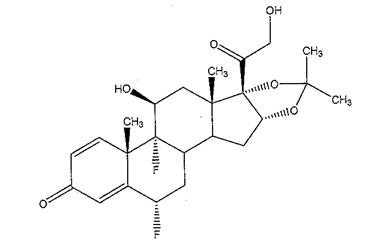
11 DESCRIPTIONILUVIEN is a sterile non-bioerodable intravitreal implant containing 0.19 mg (190 mcg) fluocinolone acetonide in a 36-month sustained-release drug delivery system. ILUVIEN is designed to release ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Corticosteroids inhibit inflammatory responses to a variety of inciting agents including multiple inflammatory cytokines. They inhibit edema, fibrin deposition ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies have not been conducted to determine the carcinogenic potential or the effect on fertility of ...
-
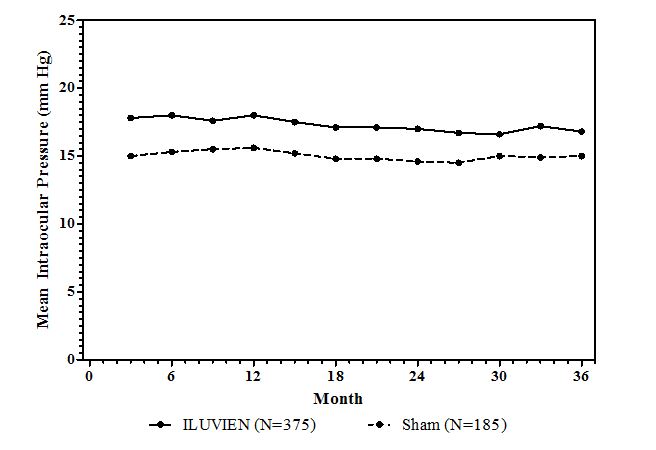
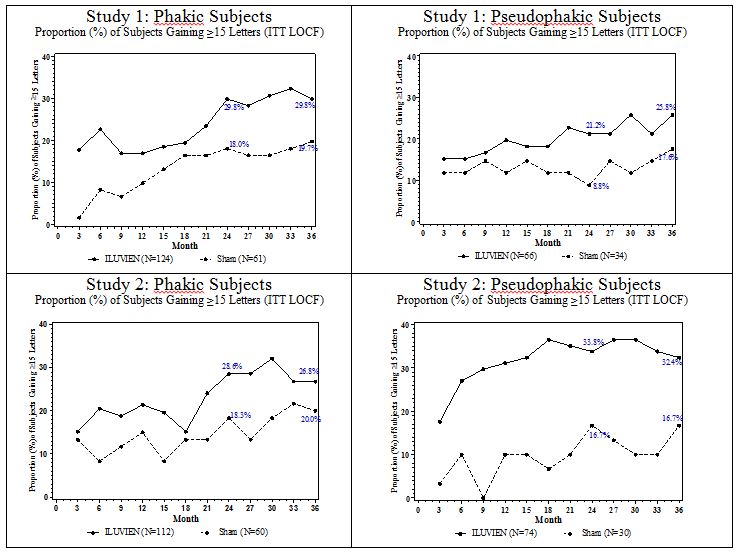
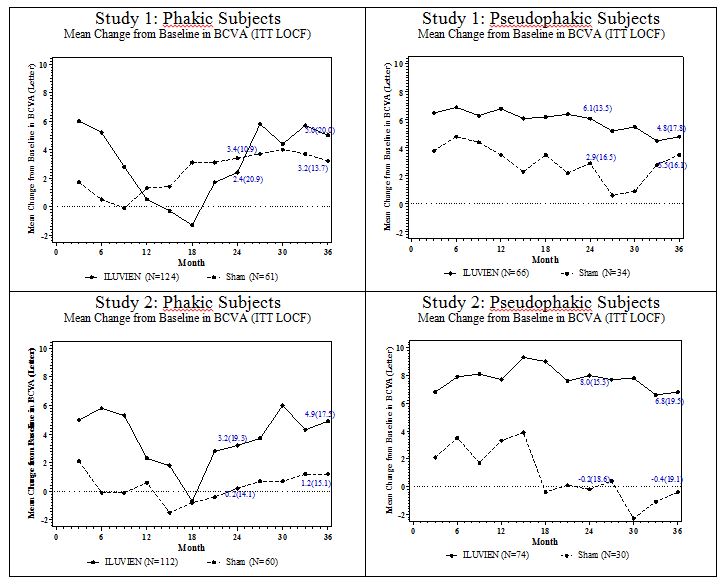
14 CLINICAL STUDIESDiabetic Macular Edema - The efficacy of ILUVIEN was assessed in two three year, randomized (2:1, active: sham), multicenter, double-masked, parallel-groups studies that enrolled patients with ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGILUVIEN® (fluocinolone acetonide intravitreal implant) 0.19 mg is supplied in a sterile, single-use preloaded applicator with a 25-gauge needle, packaged in a tray sealed with a lid inside a ...
-
17 PATIENT COUNSELING INFORMATIONSteroid-related Effects - Advise patients that a cataract may occur after treatment with ILUVIEN. If this occurs, advise patients that their vision will decrease, and they will need an ...
-
PRINCIPAL DISPLAY PANELPackage Label - Principal Display Panel – Carton
-
PRINCIPAL DISPLAY PANELPackage Label - Principal Display Panel – Lid
-
PRINCIPAL DISPLAY PANELPackage Label - Principal Display Panel – Inserter
-
INGREDIENTS AND APPEARANCEProduct Information