Label: CLONAZEPAM tablet, orally disintegrating
- NDC Code(s): 46708-364-06, 46708-365-06, 46708-366-06, 46708-367-06, view more
- Packager: Alembic Pharmaceuticals Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
BOXED WARNING
(What is this?)
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS
Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death (see WARNINGS and PRECAUTIONS).
- Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
- Limit dosages and durations to the minimum required.
- Follow patients for signs and symptoms of respiratory depression and sedation.
-
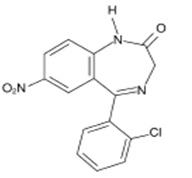
DESCRIPTIONClonazepam, USP a benzodiazepine, is available as an orally disintegrating tablet containing 0.125 mg, 0.25 mg, 0.5 mg, 1 mg or 2 mg clonazepam, USP. Each orally disintegrating tablet also ...
-
CLINICAL PHARMACOLOGYPharmacodynamics: The precise mechanism by which clonazepam exerts its antiseizure and antipanic effects is unknown, although it is believed to be related to its ability to enhance the activity ...
-
INDICATIONS AND USAGESeizure Disorders: Clonazepam orally disintegrating tablet is useful alone or as an adjunct in the treatment of the Lennox-Gastaut syndrome (petit mal variant), akinetic and myoclonic seizures ...
-
CONTRAINDICATIONSClonazepam orally disintegrating tablets are contraindicated in patients with the following conditions: History of sensitivity to benzodiazepines - Clinical or biochemical evidence of significant ...
-
WARNINGSRisks from Concomitant Use With Opioids: Concomitant use of benzodiazepines, including clonazepam orally disintegrating tablets, and opioids may result in profound sedation, respiratory ...
-
PRECAUTIONSGeneral: Worsening of Seizures: When used in patients in whom several different types of seizure disorders coexist, clonazepam orally disintegrating tablets may increase the incidence or ...
-
ADVERSE REACTIONSThe adverse experiences for clonazepam orally disintegrating tablets are provided separately for patients with seizure disorders and with panic disorder. Seizure Disorders: The most frequently ...
-
DRUG ABUSE AND DEPENDENCEControlled Substance Class: Clonazepam is a Schedule IV controlled substance. Physical and Psychological Dependence: Withdrawal symptoms, similar in character to those noted with ...
-
OVERDOSAGEHuman Experience: Symptoms of clonazepam overdosage, like those produced by other CNS depressants, include somnolence, confusion, coma and diminished reflexes. Overdose Management: Treatment ...
-
DOSAGE AND ADMINISTRATIONClonazepam is available as an orally disintegrating tablet. The orally disintegrating tablet should be administered as follows: After opening the carton, peel back the foil on the blister. Do not ...
-
HOW SUPPLIEDClonazepam orally disintegrating tablets, USP are available as follows: 0.125 mg: White to off white, round, flat-faced, beveled edge tablets, debossed with ‘L 523’ on one side and plain on ...
-
MEDICATION GUIDEClonazepam (kloe NAZ e pam) Orally Disintegrating Tablets, USP C-IV What is the most important information I should know about clonazepam orally disintegrating tablets? · Do not ...
-
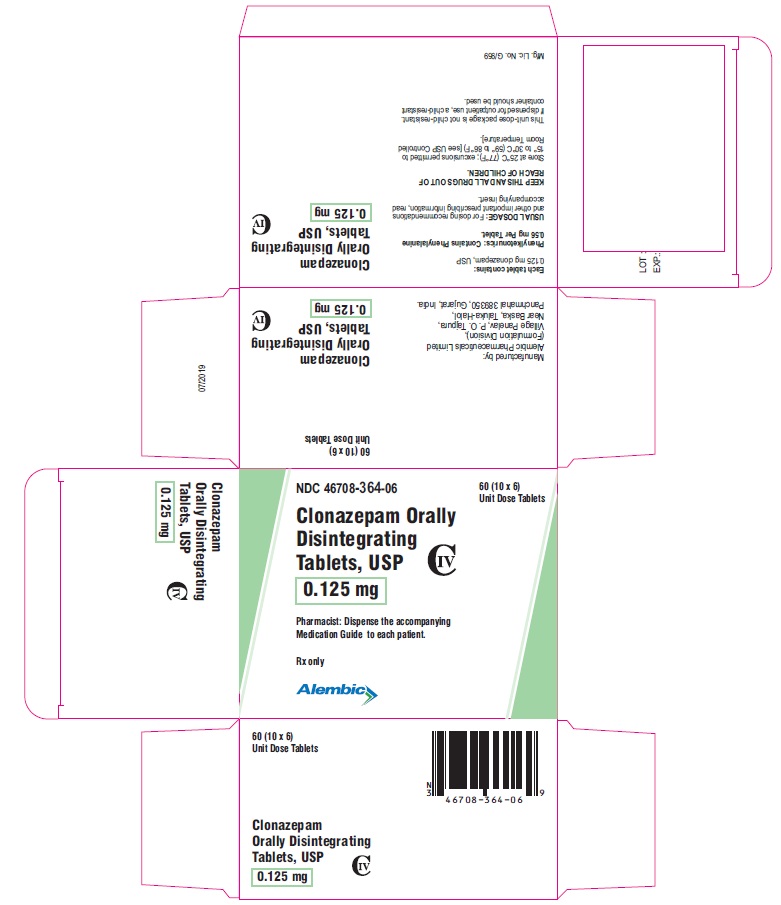
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL -0.125 mgNDC 46708-364-06 - Clonazepam Orally Disintegrating - Tablets, USP - 0.125 mg - Pharmacist: Dispense the accompanying - Medication Guide to each patient. Rx only - 60 (10 x 6) Unit Dose ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL -0.25 mgNDC 46708-365-06 - Clonazepam Orally Disintegrating - Tablets, USP - 0.25 mg - Pharmacist: Dispense the accompanying - Medication Guide to each patient. Rx only - 60 (10 x 6) Unit Dose ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL -0.5 mgNDC 46708-366-06 - Clonazepam Orally Disintegrating - Tablets, USP - 0.5 mg - Pharmacist: Dispense the accompanying - Medication Guide to each patient. Rx only - 60 (10 x 6) Unit Dose ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL -1 mgNDC 46708-367-06 - Clonazepam Orally Disintegrating - Tablets, USP - 1 mg - Pharmacist: Dispense the accompanying - Medication Guide to each patient. Rx only - 60 (10 x 6) Unit Dose ...
-
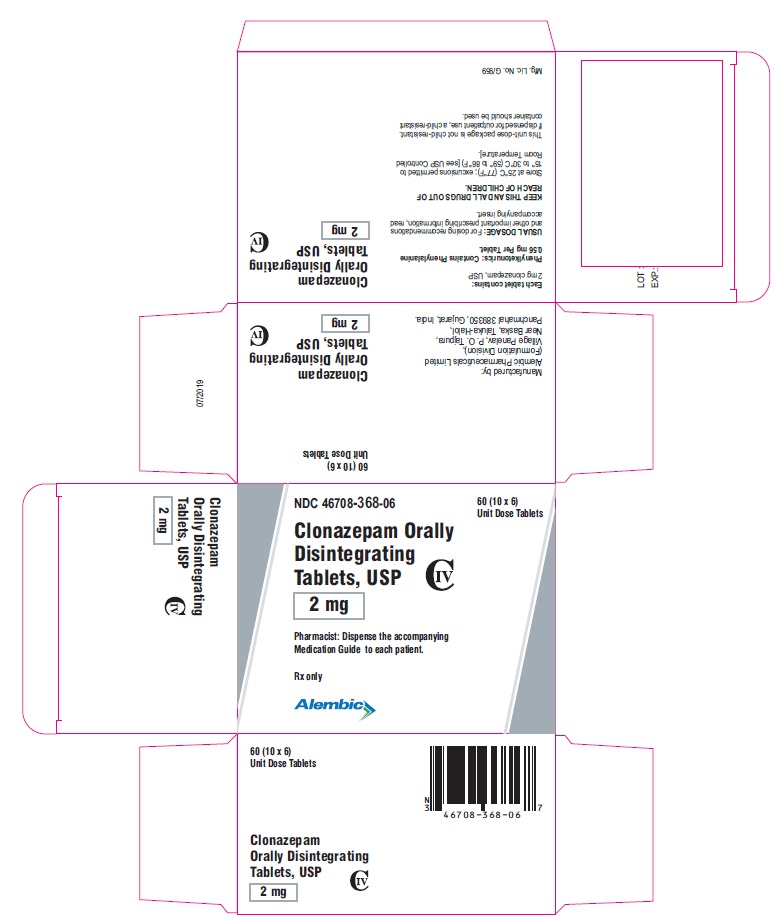
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL -2 mgNDC 46708-368-06 - Clonazepam Orally Disintegrating - Tablets, USP - 2 mg - Pharmacist: Dispense the accompanying - Medication Guide to each patient. Rx only - 60 (10 x 6) Unit Dose ...
-
INGREDIENTS AND APPEARANCEProduct Information