Label: SOFTLIPS PEARL- octinoxate, octisalate stick
- NDC Code(s): 10742-8573-1
- Packager: The Mentholatum Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
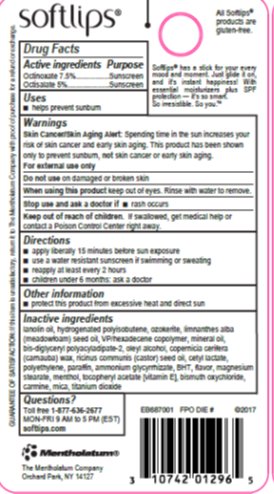
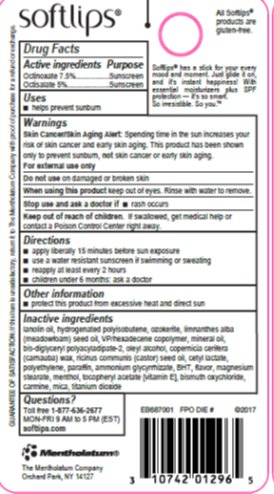
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
lanolin oil, hydrogenated polyisobutene, ozokerite, limnanthes alba (meadowfoam) seed oil, VP/hexadecene copolymer, mineral oil, bis-diglyceryl polyacyladipate-2, oleyl alcohol, copernicia cerifera (carnauba) wax, ricinus communis (castor) seed oil, cetyl lactate, polyethylene, paraffin, ammonium glycyrrhizate, BHT, flavor, magnesium stearate, menthol, tocopheryl acetate [vitamin E], bismuth oxychloride, carmine, mica, titanium dioxide
- Questions?
- Principal Display Panel
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SOFTLIPS PEARL
octinoxate, octisalate stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10742-8573 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength LANOLIN OIL (UNII: OVV5IIJ58F) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) CERESIN (UNII: Q1LS2UJO3A) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) VINYLPYRROLIDONE/HEXADECENE COPOLYMER (UNII: KFR5QEN0N9) MINERAL OIL (UNII: T5L8T28FGP) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) OLEYL ALCOHOL (UNII: 172F2WN8DV) CARNAUBA WAX (UNII: R12CBM0EIZ) CASTOR OIL (UNII: D5340Y2I9G) CETYL LACTATE (UNII: A7EVH2RK4O) PARAFFIN (UNII: I9O0E3H2ZE) AMMONIUM GLYCYRRHIZATE (UNII: 3VRD35U26C) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) MAGNESIUM STEARATE (UNII: 70097M6I30) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) CARMINIC ACID (UNII: CID8Z8N95N) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10742-8573-1 1 in 1 BLISTER PACK 02/01/2010 1 2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/01/2010 Labeler - The Mentholatum Company (002105757) Registrant - The Mentholatum Company (002105757) Establishment Name Address ID/FEI Business Operations The Mentholatum Company 002105757 manufacture(10742-8573)