Label: METHENAMINE HIPPURATE tablet
- NDC Code(s): 59746-797-01, 59746-797-65
- Packager: Jubilant Cadista Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Each white to off-white capsule shaped tablet contains 1g Methenamine Hippurate, USP which is the Hippuric Acid Salt of Methenamine (hexamethylene tetramine). The tablet also contains inactive ingredients colloidal silicon dioxide, microcrystalline cellulose, magnesium stearate, povidone and sodium starch glycolate.
-
ACTIONS
Microbiology: Methenamine hippurate tablets has antibacterial activity because the methenamine component is hydrolyzed to formaldehyde in acid urine. Hippuric acid, the other component, has some antibacterial activity and also acts to keep the urine acid. The drug is generally active against E.coli, enterococci and staphylococci. Enterobacter aerogenes is generally resistant. The urine must be kept sufficiently acid for urea-splitting organisms such as Proteus and Pseudomonas to be inhibited.
Susceptibility Testing:
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.Human Pharmacology: Within 1/2 hour after ingestion of a single 1-gram dose of methenamine hippurate, antibacterial activity is demonstrable in the urine. Urine has continuous antibacterial activity when methenamine hippurate is administered at the recommended dosage schedule of 1 gram twice daily. Over 90% of methenamine moiety is excreted in the urine within 24 hours after administration of a single 1gram dose. Similarly, the hippurate moiety is rapidly absorbed and excreted, and it reaches the urine by both tubular secretion and glomerular filtration. This action may be important in older patients or in those with some degree of renal impairment.
-
INDICATIONS
Methenamine hippurate tablets, USP is indicated for prophylactic or suppressive treatment of frequently recurring urinary tract infections when long-term therapy is considered necessary. This drug should only be used after eradication of the infection by other appropriate antimicrobial agents.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of methenamine hippurate tablets, USP and other antibacterial drugs, methenamine hippurate tablets, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
CONTRAINDICATIONS
Methenamine hippurate tablets are contraindicated in patients with renal insufficiency, severe hepatic insufficiency, or severe dehydration. Methenamine preparations should not be given to patients taking sulfonamides because some sulfonamides may form an insoluble precipitate with formaldehyde in the urine.
- WARNING
-
PRECAUTIONS
Prescribing methenamine hippurate tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
- Care should be taken to maintain an acid pH of the urine, especially when treating infections due to urea-splitting organisms such as Proteus and strains of Pseudomonas.
- In a few instances in one study, the serum transaminase levels were slightly elevated during treatment but returned to normal while the patients were still taking methenamine hippurate tablets, USP. Because of this report, it is recommended that liver function studies be performed periodically on patients taking the drug, especially those with liver dysfunction.
- Use in Pregnancy: In early pregnancy the safe use of methenamine hippurate tablets is not established. In the last trimester, safety is suggested, but not definitely proved. No adverse effects on the fetus were seen in studies in pregnant rats and rabbits.
Methenamine hippurate tablets taken during pregnancy can interfere with laboratory tests of urine estriol (resulting in unmeasurably low values) when acid hydrolysis is used in the laboratory procedure. This interference is due to the presence in the urine of methenamine and/or formaldehyde. Enzymatic hydrolysis, in place of acid hydrolysis, will circumvent this problem.
Geriatric Use
Clinical studies of methenamine hippurate tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
Methenamine hippurate tablets, USP are contraindicated in patients with renal insufficiency and severe hepatic insufficiency (see CONTRAINDICATIONS).
Information for Patients
Patients should be counseled that antibacterial drugs including methenamine hippurate tablets should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When methenamine hippurate tablets is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by methenamine hippurate tablets or other antibacterial drugs in the future.
- Care should be taken to maintain an acid pH of the urine, especially when treating infections due to urea-splitting organisms such as Proteus and strains of Pseudomonas.
-
ADVERSE REACTIONS
Minor adverse reactions have been reported in less than 3.5% of patients treated. These reactions have included nausea, upset stomach, dysuria, and rash.
To report SUSPECTED ADVERSE REACTIONS, contact Jubilant Cadista Pharmaceuticals Inc. at 1-800-313-4623 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
DOSAGE AND ADMINISTRATION
1 tablet (1.0 g) twice daily (morning and night) for adults and pediatric patients over 12 years of age. 1/2 to 1 tablet (0.5 to 1 g) twice daily (morning and night) for pediatric patients 6 to 12 years of age. Since the antibacterial activity of methenamine hippurate tablets, USP is greater in acid urine, restriction of alkalinizing foods and medications is desirable. If necessary, as indicated by urinary pH and clinical response, supplemental acidification of the urine should be instituted. The efficacy of therapy should be monitored by repeated urine cultures.
-
HOW SUPPLIED
Methenamine Hippurate Tablets USP, 1 g are white to off-white capsule shaped tablet, debossed with “L” and “59” on one side and break line (functional) on other side.
Bottle pack of 20 tablets with Child Resistant Closure, NDC 59746-797-65
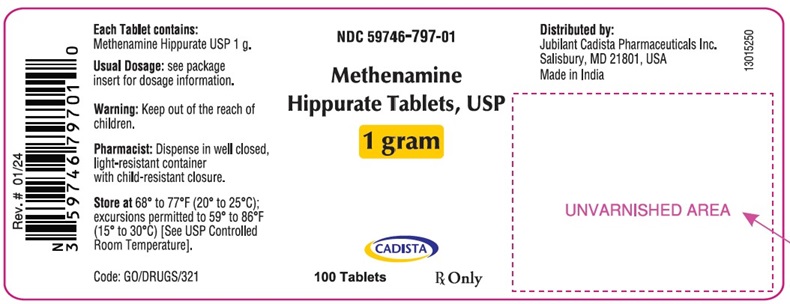
Bottle pack of 100 tablets with Child Resistant Closure, NDC 59746-797-01Store at 68° to 77°F (20° to 25°C); excursions permitted to 59° to 86°F (15° to 30°C) [See USP Controlled Room Temperature].
Dispense in well-closed, light-resistant container with child-resistant closure.Rx Only
Distributed by:
Jubilant Cadista Pharmaceuticals Inc.
Salisbury, MD 21801, USA.
Manufactured by:
Unichem Laboratories Limited,
Plot No. 10 to 18, Pilerne Industrial Estate,
Pilerne, Bardez, Goa - 403511, India (IND)
Revision 01/2024 -
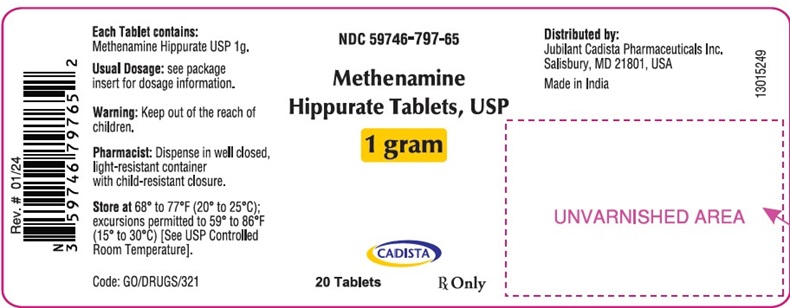
PRINCIPAL DISPLAY PANEL
NDC 59746-797-65
Methenamine
Hippurate Tablets, USP
1 gram
CADISTA
20 Tablets
Rx Only
Each Tablet contains:
Methenamine Hippurate USP 1g.
Usual Dosage: see package
insert for dosage information.
Warning: Keep out of the reach of
children.
Pharmacist: Dispense in well closed,
light-resistant container
with child-resistant closure.
Store at 68° to 77°F (20° to 25°C);
excursions permitted to 59° to 86°F
(15° to 30°C) [See USP Controlled
Room Temperature].
Code: G0/DRUGS/321
Distributed by:
Jubilant Cadista Pharmaceuticals Inc.
Salisbury, MD 21801, USA
Made in India
13015249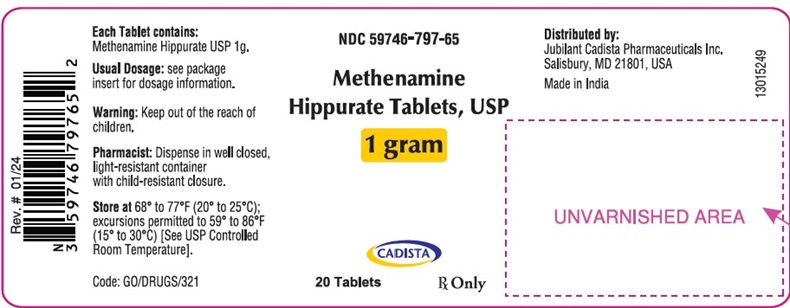
NDC 59746-797-01
100 Tablets
13015250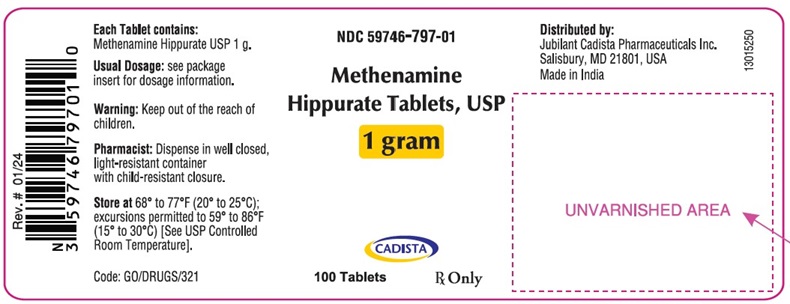
-
INGREDIENTS AND APPEARANCE
METHENAMINE HIPPURATE
methenamine hippurate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59746-797 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHENAMINE HIPPURATE (UNII: M329791L57) (METHENAMINE - UNII:J50OIX95QV) METHENAMINE HIPPURATE 1 g Inactive Ingredients Ingredient Name Strength Silicon Dioxide (UNII: ETJ7Z6XBU4) Microcrystalline Cellulose (UNII: OP1R32D61U) Magnesium Stearate (UNII: 70097M6I30) Povidone, Unspecified (UNII: FZ989GH94E) Sodium Starch Glycolate Type A (UNII: H8AV0SQX4D) Product Characteristics Color WHITE (White to off white) Score 2 pieces Shape CAPSULE Size 19mm Flavor Imprint Code L;59 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59746-797-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/24/2023 2 NDC:59746-797-65 20 in 1 BOTTLE; Type 0: Not a Combination Product 01/24/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA217675 01/24/2023 Labeler - Jubilant Cadista Pharmaceuticals Inc. (022490515) Establishment Name Address ID/FEI Business Operations Unichem Laboratories Limited 915389741 analysis(59746-797) , label(59746-797) , manufacture(59746-797) , pack(59746-797)