Label: SPEVIGO- spesolimab-sbzo injection
- NDC Code(s): 0597-0035-10, 0597-0620-10, 0597-0620-20
- Packager: Boehringer Ingelheim Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SPEVIGO safely and effectively. See full prescribing information for SPEVIGO.
SPEVIGO® (spesolimab-sbzo) injection, for subcutaneous or intravenous use
Initial U.S. Approval: 2022RECENT MAJOR CHANGES
INDICATIONS AND USAGE
SPEVIGO is an interleukin-36 receptor antagonist indicated for the treatment of generalized pustular psoriasis (GPP) in adults and pediatric patients 12 years of age and older and weighing at least 40 kg. (1)
DOSAGE AND ADMINISTRATION
Subcutaneous Dosage for Treatment of GPP When Not Experiencing a Flare
Administer a subcutaneous loading dose of 600 mg (four 150 mg injections), followed by 300 mg (two 150 mg injections) subcutaneously 4 weeks later and every 4 weeks thereafter. (2.3)
- Subcutaneous Use After Intravenous SPEVIGO for Treatment of GPP Flare: Four weeks after treatment with intravenous SPEVIGO, initiate or reinitiate subcutaneous SPEVIGO at a dose of 300 mg (two 150 mg injections) administered every 4 weeks. A loading dose is not required following treatment of a GPP flare with intravenous SPEVIGO. (2.3)
- See full prescribing information for preparation and administration instructions. (2.2, 2.5)
Intravenous Dosage for Treatment of GPP Flare
- Must be diluted before intravenous use. Administer as a single 900 mg dose by intravenous infusion over 90 minutes. If flare symptoms persist, may administer an additional intravenous 900 mg dose one week after the initial dose. (2.4)
- See full prescribing information for preparation and administration instructions and storage of the diluted solution. (2.2, 2.5)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
Severe or life-threatening hypersensitivity to spesolimab-sbzo or to any of the excipients in SPEVIGO (4)
WARNINGS AND PRECAUTIONS
- Infections: SPEVIGO may increase the risk of infections. Treatment with SPEVIGO is not recommended during any clinically important active infection. Instruct patients to seek medical advice if signs or symptoms of clinically important infection occur during or after treatment. If a clinically important active infection develops discontinue SPEVIGO until the infection resolves or is adequately treated. (5.1)
- Risk of Tuberculosis (TB): Evaluate patients for TB prior to initiating treatment with SPEVIGO. (5.2)
- Hypersensitivity and Infusion-Related Reactions: Hypersensitivity including drug reaction with eosinophilia and systemic symptoms (DRESS) and infusion-related reactions may occur. Discontinue SPEVIGO immediately and initiate appropriate treatment if a serious hypersensitivity reaction occurs. (5.3)
- Vaccinations: Avoid use of live vaccines during and for at least 16 weeks after treatment with SPEVIGO. (5.4)
ADVERSE REACTIONS
- Treatment of GPP When Not Experiencing a Flare: SPEVIGO has been associated with an increased incidence (≥9 cases per 100 patient-years) of injection site reaction, urinary tract infection, arthralgia, and pruritus. (6.1)
- Treatment of GPP flare: The most common adverse reactions (≥5%) are asthenia and fatigue, nausea and vomiting, headache, pruritus and prurigo, infusion site hematoma and bruising, and urinary tract infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Boehringer Ingelheim Pharmaceuticals, Inc. at (800) 542-6257 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 3/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Testing and Procedures Prior to Treatment Initiation
2.2 Important Administration Information
2.3 Recommended Subcutaneous Dosage for Treatment of GPP When Not Experiencing a Flare
2.4 Recommended Intravenous Dosage for Treatment of GPP Flare
2.5 Preparation and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Infections
5.2 Risk of Tuberculosis
5.3 Hypersensitivity and Infusion-Related Reactions
5.4 Vaccinations
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Testing and Procedures Prior to Treatment Initiation
Evaluate patients for active or latent tuberculosis (TB) infection. SPEVIGO initiation is not recommended in patients with active TB infection. Consider initiating treatment of latent TB prior to initiation of SPEVIGO [see Warnings and Precautions (5.2)].
Complete all age-appropriate vaccinations according to current immunization guidelines prior to initiating SPEVIGO for treatment of GPP [see Warnings and Precautions (5.4)].
2.2 Important Administration Information
Subcutaneous Use for Treatment of GPP When Not Experiencing a Flare
- SPEVIGO prefilled syringes are for subcutaneous use for treatment of GPP when not experiencing a flare.
- If required, the 600 mg subcutaneous loading dose of SPEVIGO is to be administered by a healthcare professional [see Dosage and Administration (2.3)].
- For subsequent 300 mg doses, if the healthcare professional determines that it is appropriate, a patient 12 years of age and older may self-inject or the caregiver may administer SPEVIGO after proper training in subcutaneous injection technique. In pediatric patients 12 to 17 years of age, administer SPEVIGO under the supervision of an adult.
- If a patient experiences a GPP flare while receiving subcutaneous SPEVIGO, the GPP flare may be treated with intravenous SPEVIGO [see Dosage and Administration (2.4)].
Intravenous Use for Treatment of GPP Flare
- SPEVIGO vials are for intravenous use for treatment of GPP flare.
- Intravenous infusion of SPEVIGO is only to be administered by a healthcare professional in a healthcare setting. Prepare SPEVIGO intravenous infusion by diluting SPEVIGO single-dose vials [see Dosage and Administration (2.5)].
- Do not mix SPEVIGO with other medicinal products.
2.3 Recommended Subcutaneous Dosage for Treatment of GPP When Not Experiencing a Flare
The recommended dosage of SPEVIGO for treatment of GPP when not experiencing a flare in adults and pediatric patients 12 years of age and older and weighing at least 40 kg is a loading dose of 600 mg (four 150 mg injections) followed by 300 mg (two 150 mg injections) administered subcutaneously 4 weeks later and every 4 weeks thereafter.
Initiating or Reinitiating Subcutaneous SPEVIGO After Treatment of a GPP Flare with Intravenous SPEVIGO
Four weeks after treatment of a GPP flare with intravenous SPEVIGO [see Dosage and Administration (2.4)], initiate or reinitiate subcutaneous SPEVIGO for treatment of GPP at a dose of 300 mg (two 150 mg injections) administered every 4 weeks. A subcutaneous loading dose is not required following treatment of a GPP flare with intravenous SPEVIGO.
2.4 Recommended Intravenous Dosage for Treatment of GPP Flare
The recommended dosage of SPEVIGO for treatment of GPP flare in adults and pediatric patients 12 years of age and older and weighing at least 40 kg is a single 900 mg dose administered by intravenous infusion over 90 minutes.
If GPP flare symptoms persist, an additional intravenous 900 mg dose (over 90 minutes) may be administered one week after the initial dose.
2.5 Preparation and Administration Instructions
Parenteral drug products should be inspected visually for particulate matter and discoloration, whenever solution and container permits. SPEVIGO is a colorless to slightly brownish-yellow, clear to slightly opalescent solution. Do not use if the solution is cloudy, discolored, or contains large or colored particulates.
Prefilled Syringe (Subcutaneous Use for Treatment of GPP When Not Experiencing a Flare)
- Before subcutaneous injection, remove SPEVIGO prefilled syringes from the refrigerator and allow SPEVIGO to reach room temperature (15 to 30 minutes) without removing the needle cap.
- Administer SPEVIGO subcutaneously in the upper thighs or abdomen. Do not inject into areas where the skin is tender, bruised, erythematous, indurated, or scarred.
- Choose a different injection site for each injection, at least 1 inch away from the other injection sites. Alternate between the upper thigh and abdomen injection sites for each complete dose.
- For a complete 300 mg dose, two 150 mg/mL prefilled syringes are required to be injected, one right after the other.
- If a dose is missed, administer the dose as soon as possible. Thereafter, resume dosing at the regular scheduled time.
- The SPEVIGO "Instructions for Use" contains more detailed instructions on the preparation and administration of SPEVIGO [see Instructions for Use].
Vial (Intravenous Use for Treatment of GPP Flare)
Preparation
- SPEVIGO solution for intravenous infusion must be diluted before use.
- Use aseptic technique to prepare the solution for infusion.
- Draw and discard 15 mL from a 100 mL container of sterile 0.9% Sodium Chloride Injection.
- Slowly replace with 15 mL of SPEVIGO (complete content from two vials of 450 mg/7.5 mL).
- Mix gently before use.
- Use the diluted SPEVIGO solution immediately. If not administered immediately, refrigerate the diluted solution at 2°C to 8°C (36°F to 46°F) for no more than 4 hours. Protect from light.
Administration
- Administer SPEVIGO as a continuous intravenous infusion through an intravenous line containing a sterile, non-pyrogenic, low protein binding in-line filter (pore size of 0.2 micron) over 90 minutes.
- A pre-existing intravenous line may be used for administration of SPEVIGO. The line must be flushed with sterile 0.9% Sodium Chloride Injection prior to and at the end of infusion. No other infusion should be administered in parallel via the same intravenous access.
- If the infusion is slowed or temporarily stopped, the total infusion time (including stop time) should not exceed 180 minutes [see Warnings and Precautions (5.3)].
- No incompatibilities have been observed between SPEVIGO and infusion sets composed of polyvinylchloride (PVC), polyethylene (PE), polypropylene (PP), polybutadiene and polyurethane (PUR), and in-line filter membranes composed of polyethersulfone (PES, neutral and positively charged) and positively charged polyamide (PA).
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
SPEVIGO is contraindicated in patients with severe or life-threatening hypersensitivity to spesolimab-sbzo or to any of the excipients in SPEVIGO. Reported hypersensitivity reactions have included drug reaction with eosinophilia and systemic symptoms (DRESS) [see Warnings and Precautions (5.3) and Adverse Reactions (6.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Infections
SPEVIGO may increase the risk of infections. During the one-week placebo-controlled period in the Effisayil-1 trial, infections were reported in 14% of subjects treated with SPEVIGO compared with 6% of subjects treated with placebo [see Adverse Reactions (6.1)].
In patients with a chronic infection or a history of recurrent infection, consider the potential risks and expected clinical benefits of treatment prior to prescribing SPEVIGO. Treatment with SPEVIGO is not recommended in patients with any clinically important active infection until the infection resolves or is adequately treated. Instruct patients to seek medical advice if signs or symptoms of clinically important infection occur during or after treatment with SPEVIGO. If a patient develops a clinically important active infection, discontinue SPEVIGO therapy until the infection resolves or is adequately treated.
5.2 Risk of Tuberculosis
Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with SPEVIGO. Avoid use of SPEVIGO in patients with active TB infection.
Consider initiating anti-TB therapy prior to initiating SPEVIGO in patients with latent TB or a history of TB in whom an adequate course of treatment cannot be confirmed. Monitor patients for signs and symptoms of active TB during and after SPEVIGO treatment.
5.3 Hypersensitivity and Infusion-Related Reactions
SPEVIGO-associated hypersensitivity reactions may include immediate reactions such as anaphylaxis and delayed reactions such as drug reaction with eosinophilia and systemic symptoms (DRESS).
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) has been reported in clinical trials with SPEVIGO in subjects with GPP [see Adverse Reactions (6.1)].
If a patient develops signs of anaphylaxis or other serious hypersensitivity, discontinue SPEVIGO immediately and initiate appropriate treatment. SPEVIGO is contraindicated in patients with severe or life-threatening hypersensitivity to spesolimab-sbzo or to any of the excipients in SPEVIGO [see Contraindications (4)].
If a patient develops mild or moderate hypersensitivity during an intravenous infusion or other infusion-related reactions, stop SPEVIGO infusion and consider appropriate medical therapy (e.g., systemic antihistamines and/or corticosteroids). Upon resolution of the reaction, the infusion may be restarted at a slower infusion rate with gradual increase to complete the infusion.
5.4 Vaccinations
Prior to initiating SPEVIGO for treatment of GPP, complete all age-appropriate vaccinations according to current immunization guidelines.
Avoid use of live vaccines in patients during and for at least 16 weeks after treatment with SPEVIGO. No specific studies have been conducted in SPEVIGO-treated patients who have recently received live viral or live bacterial vaccines.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Infections [see Warnings and Precautions (5.1)]
- Hypersensitivity and Infusion-Related Reactions [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adverse Reactions with Intravenous SPEVIGO for Treatment of GPP Flare (Study Effisayil-1)
SPEVIGO was studied in Study Effisayil-1, a randomized, double-blind, placebo-controlled study comparing a single intravenous 900 mg dose of SPEVIGO (n=35) with placebo (n=18) in subjects with generalized pustular psoriasis (GPP) flare. Subjects in either treatment group who continued to experience flare symptoms at Week 1 were eligible to receive a single open-label intravenous dose of 900 mg of SPEVIGO (second dose and first dose for subjects in the SPEVIGO and placebo groups, respectively). At Week 1, 12 (34%) subjects and 15 subjects (83%) in the SPEVIGO and placebo groups, respectively, received open-label SPEVIGO. After Week 1 to Week 12, subjects in either treatment group whose GPP flare reoccurred after achieving a clinical response were eligible to receive a single open-label rescue intravenous dose of 900 mg of SPEVIGO, with a maximum of 3 total doses of SPEVIGO throughout the study. Six subjects received a single open-label rescue dose of SPEVIGO. Thirty-six subjects received 1 dose of SPEVIGO, 13 subjects received 2 doses of SPEVIGO, and 2 subjects received 3 doses of SPEVIGO throughout the study [see Clinical Studies (14)].
Subjects ranged in age from 21 to 69 years (mean age of 43 years); 45% were White and 55% were Asian; and 68% were female.
Table 1 summarizes selected adverse reactions that occurred at a rate of at least 1% and at a higher rate in the intravenous SPEVIGO group than in the placebo group through Week 1.
Table 1 Selected Adverse Reactions Occurring in ≥1% of the Intravenous SPEVIGO Group and More Frequently than in the Placebo Group through Week 1 in Subjects with GPP Flare (Study Effisayil-1) Adverse Reaction Intravenous SPEVIGO
N = 35
n (%)Placebo
N = 18
n (%)Asthenia and Fatigue 3 (9) 0 Nausea and Vomiting 3 (9) 1 (6) Headache 3 (9) 1 (6) Pruritus and prurigo 2 (6) 0 Infusion site hematoma and bruising 2 (6) 0 Urinary tract infection 2 (6) 0 Bacteremia 1 (3) 0 Bacteriuria 1 (3) 0 Cellulitis 1 (3) 0 Herpes dermatitis and oral herpes 1 (3) 0 Upper respiratory tract infection 1 (3) 0 Dyspnea 1 (3) 0 Eye edema 1 (3) 0 Urticaria 1 (3) 0 Specific Adverse Reactions
Infections
The most frequent adverse reactions that occurred in subjects treated with intravenous SPEVIGO were infections. During the 1-week placebo-controlled period in Study Effisayil-1, infections were reported in 14% of subjects treated with SPEVIGO compared with 6% of subjects treated with placebo. Serious infection (urinary tract infection) was reported in 1 subject (3%) treated with SPEVIGO and no subjects treated with placebo. Infections observed through Week 1 in Study Effisayil-1 in subjects treated with SPEVIGO were mild (29%) to moderate (71%).
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
Two cases of DRESS were reported in Study Effisayil-1 in subjects with GPP who were treated with intravenous SPEVIGO. RegiSCAR DRESS validation scoring (with the following categories: "no", "possible", "probable", or "definite" DRESS) was applied to the reported cases. Reported cases were assessed as "no DRESS" and "possible DRESS".
Safety through Week 12 and 17
In Study Effisayil-1, additional adverse reactions that occurred through Week 12 in subjects treated with 1 single intravenous dose of randomized SPEVIGO were mild to moderate infections: device-related infection (3%), subcutaneous abscess (3%), furuncle (3%), and influenza (3%).
Additional adverse reactions that occurred through Week 17 in subjects treated with a single intravenous dose of open-label SPEVIGO at Week 1 (second dose and first dose for subjects in the SPEVIGO and placebo groups, respectively) were mild to moderate infections: otitis externa (7%), vulvovaginal candidiasis (4%), vulvovaginal mycotic infection (4%), latent tuberculosis (4%), diarrhea (11%), and gastritis (4%). No new adverse reactions were identified for up to 16 weeks in subjects treated with a single intravenous dose of open-label rescue SPEVIGO from Week 1 to Week 12 (range 1-3 total doses).
Adverse Reactions with Subcutaneous SPEVIGO for Treatment of GPP When Not Experiencing a Flare (Study Effisayil-2)
Subcutaneous treatment with SPEVIGO was studied in Study Effisayil-2, a randomized, placebo-controlled, double-blind, parallel group study evaluating three dosages of SPEVIGO or placebo in subjects with generalized pustular psoriasis (GPP). Subjects were randomized (1:1:1:1) to receive a 600 mg loading dose (LD) of SPEVIGO followed by 300 mg every 4 weeks (n=30), one of two other dosages of SPEVIGO, or placebo (n=30) for up to 48 weeks [see Clinical Studies (14)].
Subjects ranged in age from 14 to 75 years (mean age was 40 years); 64% of subjects were Asian and 36% were White; 62% of subjects were female.
Regarding the exposure-adjusted incidence rates for subjects on randomized treatment prior to receiving rescue treatment for flare or completing trial without a flare, the rate per 100-patient years for injection site reaction (including erythema, pain, swelling, induration, urticaria, and warmth at the injection site) was 31.6 for the subcutaneous SPEVIGO cohort (600 mg LD followed by 300 mg every 4 weeks) compared to 12.7 for the placebo cohort.
Regarding the exposure-adjusted incidence rates for subjects on randomized treatment prior to receiving rescue treatment for flare or completing trial without a flare, the rate per 100-patient years for urinary tract infection was 18 for the subcutaneous SPEVIGO cohort (600 mg LD followed by 300 mg every 4 weeks) compared to 0 for the placebo cohort.
Regarding the exposure-adjusted incidence rates for subjects on randomized treatment prior to receiving rescue treatment for flare or completing trial without a flare, the rate per 100-patient years for pruritus was 8.8 for the subcutaneous SPEVIGO cohort (600 mg LD followed by 300 mg every 4 weeks) compared to 0 for the placebo cohort.
Regarding the exposure-adjusted incidence rates for subjects on randomized treatment prior to receiving rescue treatment for flare or completing trial without a flare, the rate per 100-patient years for arthralgia was 13.3 for the subcutaneous SPEVIGO cohort (600 mg LD followed by 300 mg every 4 weeks) compared to 6 for the placebo cohort.
For subjects on randomized treatment prior to receiving rescue treatment for flare or completing trial without flare, there were 3 subjects who discontinued subcutaneous SPEVIGO in the subcutaneous SPEVIGO cohort (600 mg LD followed by 300 mg every 4 weeks) due to treatment emergent adverse events of psoriasis compared to no subjects in the placebo cohort who discontinued placebo for any treatment emergent adverse event.
Safety In Study Effisayil-2 after flare
In Effisayil-2, subjects who experienced a GPP flare and received at least one dose of an open-label single intravenous 900 mg dose of SPEVIGO were treated with open-label subcutaneous SPEVIGO 300 mg. These subjects (n=19) received subcutaneous dosing at every 12 weeks which could have been increased to every 4 weeks based on GPPPGA total score or pustulation sub score increased by ≥1 from any previous OL maintenance visit. The reported safety profile of open-label subcutaneous SPEVIGO use after treatment of GPP flare with open-label intravenous SPEVIGO use was consistent with the safety profiles of use of SPEVIGO from Trial Effisayil-1 and randomized controlled data from Trial Effisayil-2.
Clinical Development of Spesolimab-sbzo
Guillain-Barre syndrome
Among approximately 835 subjects exposed to spesolimab-sbzo during clinical development, Guillain-Barre syndrome (GBS) was reported in 3 subjects who received various doses of spesolimab-sbzo via various methods of administration in clinical studies for unapproved indications.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited data on the use of SPEVIGO in pregnant women are insufficient to inform a drug-associated risk of adverse pregnancy-related outcomes. Human IgG is known to cross the placental barrier; therefore, SPEVIGO may be transmitted from the mother to the developing fetus. In an animal reproduction study, intravenous administration of a surrogate antibody against IL36R in mice during the period of organogenesis did not elicit any reproductive toxicity (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Embryo-fetal development and pre- and postnatal development toxicity studies were performed in mice using a surrogate mouse specific IL36R antagonist monoclonal antibody. In the embryo-fetal development study, the surrogate was administered intravenously at doses up to 50 mg/kg to pregnant female mice twice weekly during the period of organogenesis. The surrogate was not associated with embryo-fetal lethality or fetal malformations. In the pre- and postnatal development toxicity study, the surrogate was administered intravenously at doses up to 50 mg/kg to pregnant female mice twice weekly from gestation day 6 through lactation day 18. There were no maternal effects observed. There were no treatment-related effects observed on postnatal developmental, neurobehavioral, or reproductive performance of offspring.
8.2 Lactation
Risk Summary
There are no data on the presence of spesolimab-sbzo in human milk, the effects on the breastfed infant, or the effects on milk production. Spesolimab-sbzo is a monoclonal antibody and is expected to be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for SPEVIGO and any potential adverse effects on the breastfed infant from SPEVIGO or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of SPEVIGO for the treatment of GPP have been established in pediatric patients 12 years of age and older and weighing at least 40 kg. Use of SPEVIGO for this indication is supported by data from a randomized, placebo-controlled study which included 6 pediatric subjects 14 to 17 years of age with a history of GPP treated with subcutaneous SPEVIGO (Study Effisayil-2) and evidence from an adequate and well-controlled study of intravenous SPEVIGO in adults with GPP (Study Effisayil-1), with additional pharmacokinetic analyses showing similar drug exposure levels in adults and pediatric subjects 12 years of age and older and weighing 40 kg or more [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)].
The safety and effectiveness of SPEVIGO in pediatric patients younger than 12 years of age or in pediatric patients weighing less than 40 kg have not been established.
8.5 Geriatric Use
There were 2 (6%) intravenous SPEVIGO-treated subjects 65 to 74 years of age and no subjects 75 years of age or older in Study Effisayil-1. There were 6 (7%) subcutaneous SPEVIGO-treated subjects 65 to 74 years of age and 1 (1%) subject 75 years of age in Study Effisayil-2. Clinical studies of SPEVIGO did not include sufficient numbers of subjects 65 years of age and older to determine whether they respond differently from younger adult subjects.
-
11 DESCRIPTION
Spesolimab-sbzo, an interleukin-36 receptor antagonist, is a humanized monoclonal IgG1 antibody (mAb) against human IL-36R produced in Chinese hamster ovary (CHO) cells by recombinant DNA technology. Spesolimab-sbzo has a molecular weight of approximately 146 kDa.
SPEVIGO (spesolimab-sbzo) injection is a sterile, preservative-free, colorless to slightly brownish-yellow, clear to slightly opalescent solution supplied in a single-dose prefilled syringe for subcutaneous use. Each 1 mL prefilled syringe contains 150 mg spesolimab-sbzo, arginine hydrochloride (5.3 mg), glacial acetic acid (0.32 mg), polysorbate 20 (0.40 mg), sodium acetate (3.25 mg), sucrose (51.4 mg), and Water for Injection, USP with a pH of 5.2 – 5.8.
SPEVIGO (spesolimab-sbzo) injection is a sterile, preservative-free, colorless to slightly brownish-yellow, clear to slightly opalescent solution supplied in a single-dose vial for intravenous use. Each 7.5 mL vial contains 450 mg spesolimab-sbzo, arginine hydrochloride (39.5 mg), glacial acetic acid (2.4 mg), polysorbate 20 (3.0 mg), sodium acetate (24.5 mg), sucrose (386 mg), and Water for Injection, USP with a pH of 5.0-6.0.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Spesolimab-sbzo is a humanized monoclonal immunoglobulin G1 antibody that inhibits interleukin-36 (IL-36) signaling by specifically binding to the IL36R. Binding of spesolimab-sbzo to IL36R prevents the subsequent activation of IL36R by its ligands (IL-36 α, β and γ) and downstream activation of pro-inflammatory and pro-fibrotic pathways. The precise mechanism linking reduced IL36R activity and the treatment of flares of GPP is unclear.
12.2 Pharmacodynamics
The pharmacodynamics of SPEVIGO in the treatment of patients with GPP have not been fully characterized.
12.3 Pharmacokinetics
A population pharmacokinetic model was developed based on data collected from healthy subjects, patients with GPP, and patients with other diseases. After a single intravenous dose of 900 mg of SPEVIGO, the population PK model-estimated AUC0-∞ (95% CI) and Cmax (95% CI) in a typical anti-drug antibody (ADA)-negative patient with GPP were 4750 (4510, 4970) mcg∙day/mL and 238 (218, 256) mcg/mL, respectively. After subcutaneous SPEVIGO 600 mg LD followed by 300 mg every 4 weeks, the mean steady-state trough concentration ranged from 33.4 mcg/mL to 42.3 mcg/mL.
When administered intravenously, spesolimab-sbzo AUC increased dose-proportionally from 0.3 to 20 mg/kg, and CL and terminal half-life were independent of dose. Following subcutaneous single dose administration, spesolimab-sbzo exposure increased slightly more than dose-proportionally across the dose range of 150 mg to 600 mg due to increased bioavailability at higher doses.
Absorption
Following subcutaneous single dose administration of SPEVIGO in healthy volunteers, peak plasma concentrations were achieved between 5.5 to 7 days after dosing. After subcutaneous administration in the abdomen, absolute bioavailability (90% CI) was slightly higher at higher doses with estimated values of 58 (51, 66)%, 65 (60, 69)%, and 72 (65, 79)% at 150 mg, 300 mg, and 600 mg, respectively. Based on limited data, absolute bioavailability (90% CI) in the thigh was approximately 85 (77, 94)% following a subcutaneous dose of 300 mg SPEVIGO.
Distribution
Based on the population pharmacokinetic analysis, the typical total volume of distribution at steady state was 6.4 L.
Elimination
Specific Populations
Age, Gender, and Race
Based on population pharmacokinetic analyses, age, gender, and race did not have an effect on the pharmacokinetics of spesolimab-sbzo.
Hepatic and Renal Impairment
As a monoclonal antibody, spesolimab-sbzo is not expected to undergo hepatic or renal elimination. No formal study of the effect of hepatic or renal impairment on the pharmacokinetics of spesolimab-sbzo was conducted.
Body Weight
Spesolimab-sbzo concentrations were increased in subjects with lower body weight and decreased in subjects with higher body weight. The clinical impact of body weight on spesolimab-sbzo plasma concentrations is unknown.
Pediatric Patients
The pharmacokinetics of spesolimab-sbzo have not been studied in pediatric subjects below the age of 12 years. The plasma pharmacokinetics of spesolimab-sbzo observed in pediatric subjects 12 years of age and older and weighing 40 kg or more following subcutaneous administration were consistent with that observed in adults.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of spesolimab-sbzo or of other spesolimab products.
In subjects with GPP treated with intravenous SPEVIGO in Study Effisayil-1, ADAs formed with a median onset of 2.3 weeks. Following administration of 900 mg intravenous SPEVIGO, (12/50) 24% of subjects had a maximum ADA titer greater than 4000 and were neutralizing antibody (Nab)-positive by the end of the study (Weeks 12 to 17). In Study Effisayil-2, ADAs formed with a median onset of 8 weeks. Following administration of a 600 mg subcutaneous LD of SPEVIGO followed by 300 mg every 4 weeks for a total duration of 48 weeks, 24% of subjects had a maximum ADA titer greater than 4000 and were Nab-positive.
In Study Effisayil-1 following intravenous SPEVIGO, females appeared to have higher immunogenicity response; the percentage of subjects with ADA titer greater than 4000 was 30% in females, and 12% in males, respectively. In Study Effisayil-2 following administration of subcutaneous SPEVIGO, the data on immunogenicity response in males versus females were inconclusive.
Anti-Drug Antibody Effects on Pharmacokinetics
In subjects with ADA titers below 4000, there was no apparent impact on spesolimab-sbzo pharmacokinetics. In some subjects with ADA titer values greater than 4000, plasma spesolimab-sbzo concentrations were significantly reduced after reaching this ADA titer.
In Study Effisayil-1, there are limited data on the impact of ADAs on safety and efficacy upon retreatment as the majority of subjects did not experience a subsequent, new flare in an open-label extension study.
In subjects who received SPEVIGO 600 mg subcutaneous LD followed by 300 mg every 4 weeks in Study Effisayil-2, the mean trough concentrations beginning at Week 8 in ADA-positive subjects with titers greater than 4000 were approximately 77% to 107% of trough concentrations in subjects who were ADA-negative or ADA-positive with titer values less than 4000. After subcutaneous administration of SPEVIGO, there appears to be no impact of ADA presence on efficacy. This observation is based on limited numbers of ADA-positive subjects (N = 11). There are insufficient data to assess whether there is a clinically significant effect of ADAs on the safety of spesolimab-sbzo.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity and mutagenicity studies have not been conducted with spesolimab-sbzo.
No adverse effects on fertility were observed in male or female mice that were intravenously administered a surrogate antibody to IL36R at doses up to 50 mg/kg twice weekly.
-
14 CLINICAL STUDIES
Intravenous SPEVIGO for Treatment of GPP Flare (Study Effisayil-1)
A randomized, double-blind, placebo-controlled study (Study Effisayil-1) [NCT03782792] was conducted to evaluate the clinical efficacy and safety of intravenous SPEVIGO in adult subjects with flares of generalized pustular psoriasis (GPP). Subjects were randomized if they had a flare of GPP of moderate-to-severe intensity, as defined by:
- A Generalized Pustular Psoriasis Physician Global Assessment (GPPPGA) total score of at least 3 (moderate) [the total GPPPGA score ranges from 0 (clear) to 4 (severe)],
- The presence of fresh pustules (new appearance or worsening of pustules),
- GPPPGA pustulation sub score of at least 2 (mild), and
- At least 5% of body surface area covered with erythema and the presence of pustules.
Subjects were required to discontinue systemic and topical therapy for GPP prior to receiving study drug.
A total of 53 subjects were randomized (2:1) to receive a single intravenous dose of 900 mg SPEVIGO (N=35) or placebo (N=18) (administered over 90 minutes) during the double-blind portion of the study.
The study population consisted of 32% male and 68% female. The mean age was 43 years (range: 21 to 69 years); 55% of subjects were Asian and 45% were White. For ethnicity, there were no subjects that identified as Hispanic or Latino in the study. Most subjects included in the study had a GPPPGA pustulation sub score of 3 (43%) or 4 (36%), and subjects had a GPPPGA total score of 3 (81%) or 4 (19%). In this study, 25% of subjects had been previously treated with biologic therapy for GPP. At baseline acute flare, of the subjects with white blood cell count (WBC) assessments, 45% and 31% of subjects in the intravenous SPEVIGO and placebo groups, respectively, had (WBC) >12 × 109/L. Seventeen percent and 11% of subjects in the intravenous SPEVIGO and placebo groups, respectively, had temperature >38° Celsius. Of the subjects with WBC assessments, 12% and 6% of subjects in the SPEVIGO and placebo groups, respectively, had both WBC >12 × 109/L and temperature >38° Celsius [see Adverse Reactions (6.1)].
The primary endpoint of the study was the proportion of subjects with a GPPPGA pustulation sub score of 0 (indicating no visible pustules) at Week 1 after treatment.
Clinical Response
The results of the primary endpoint are presented in Table 2.
Table 2 GPPPGA Pustulation Sub Score at Week 1 in Adult Subjects with Flares of GPP in Study Effisayil-1 (Intravenous SPEVIGO) Intravenous SPEVIGO
(N=35)Placebo
(N=18)GPPPGA = Generalized Pustular Psoriasis Physician Global Assessment Subjects achieving a GPPPGA pustulation sub score of 0, n (%) 19 (54) 1 (6) Risk difference versus placebo, % (95% CI) 49 (21, 67) In Study Effisayil-1, subjects in either treatment group who continued to experience flare symptoms at Week 1 were eligible to receive a single open-label intravenous dose of 900 mg of SPEVIGO (second dose and first dose for subjects in the SPEVIGO and placebo groups, respectively). At Week 1, 12 (34%) subjects and 15 subjects (83%) in the intravenous SPEVIGO and placebo groups, respectively, received open-label SPEVIGO. In subjects who were randomized to intravenous SPEVIGO and received an open-label dose of SPEVIGO at Week 1, 5 (42%) subjects had a GPPPGA pustulation sub score of 0 at Week 2 (one week after their second dose of SPEVIGO).
This study did not include sufficient numbers of subjects to determine if there are differences in response according to biological sex, age, race, baseline GPPPGA pustulation sub score, and baseline GPPPGA total score.
Subcutaneous SPEVIGO for Treatment of GPP When Not Experiencing a Flare (Study Effisayil-2)
A randomized, double-blind, placebo-controlled study (Study Effisayil-2) [NCT04399837] evaluated the efficacy and safety of SPEVIGO for subcutaneous administration in adults and pediatric subjects (12 years of age and older and weighing at least 40 kg) with a history of at least two GPP flares of moderate-to-severe intensity in the past. Subjects were randomized if they had a GPPPGA total score of 0 or 1 at screening and randomization. Subjects were required to discontinue systemic and topical therapy for GPP prior to or at randomization. These subjects must have had a history of flaring while on concomitant treatment for GPP or a history of flaring upon dose reduction or discontinuation of these concomitant medications.
A total of 123 subjects were randomized (1:1:1:1) to one of four treatment arms:
- SPEVIGO: 600 mg subcutaneous loading dose (LD) followed by 300 mg subcutaneously every 4 weeks
- SPEVIGO: 600 mg subcutaneous LD followed by 300 mg subcutaneously every 12 weeks
- SPEVIGO: 300 mg subcutaneous LD followed by 150 mg subcutaneously every 12 weeks
- Placebo: subcutaneous LD followed by subcutaneous treatment every 4 weeks
While a 600 mg LD dose of SPEVIGO followed by 300 mg every 12 weeks dosage and a 300 mg LD of SPEVIGO followed by 150 mg every 12 weeks dosage were studied in Study Effisayil-2, these dosages are not approved. The recommended dosage of SPEVIGO for treatment of GPP when not experiencing a flare is a subcutaneous LD of 600 mg followed by 300 mg subcutaneously, administered every 4 weeks [see Dosage and Administration (2.3)]. The results summarized in Table 3 are those for the recommended dosage regimen.
The study population was 38% male and 62% female. The mean age was 40 years old (range: 14 to 75 years) with 8 (7%) pediatric subjects (2 per treatment arm); 64% of subjects were Asian and 36% were White. For ethnicity, 6% of subjects identified as Hispanic or Latino. Subjects included in the study had a GPPPGA pustulation sub score of 1 (28%) or 0 (72%), and subjects had a GPPPGA total score of 1 (86%) or 0 (14%). At the time of randomization, 75% of subjects were treated with systemic therapy for GPP, which was discontinued at the start of the randomized study treatment.
Subjects who experienced a GPP flare were eligible to receive up to two open-label intravenous doses of 900 mg SPEVIGO [see Dosage and Administration (2.4)]. Two (7%) subjects in the subcutaneous SPEVIGO 600 mg LD/300 mg every 4 weeks arm and 15 (48%) subjects in the placebo arm received intravenous SPEVIGO for treatment of GPP flare.
The primary endpoint of the study was the time to the first GPP flare up to Week 48 (defined by a GPPPGA pustulation sub score of ≥2 and an increase in GPPPGA total score by ≥2 from baseline). The key secondary endpoint was the occurrence of at least one GPP flare up to Week 48.
Clinical Response
The number of subjects who experienced at least one GPP flare and the time to first GPP flare are presented in Table 3 and Figure 1.
Table 3 Occurrence of at Least One GPP Flare up to Week 48 in Adults and Pediatric Subjects (12 Years of Age and Older and Weighing At Least 40 kg) With a History of GPP in Study Effisayil-2 (Subcutaneous SPEVIGO) Subcutaneous SPEVIGO 600 mg LD, followed by 300 mg every 4 weeks
(N=30)Placebo
(N=31)*The use of intravenous SPEVIGO treatment or investigator-prescribed standard of care to treat GPP worsening were considered as onset of GPP flare Subjects with GPP flares, n (%)* 3 (10) 16 (52) Risk difference for GPP flare occurrence vs placebo,% (95% CI) -39
(-62, -16)Figure 1 Time to First GPP Flare up to Week 48 in Adults and Pediatric Subjects (12 Years of Age and Older and Weighing At Least 40 kg) With a History of GPP in Study Effisayil-2 (Subcutaneous SPEVIGO)
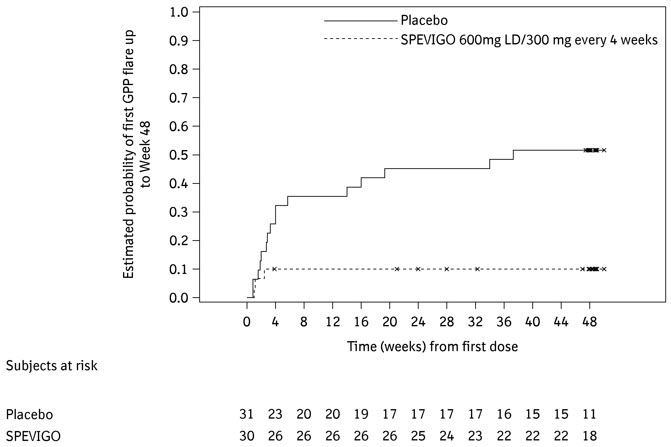
The results of the primary and key secondary endpoints were generally consistent across subgroups including biological sex, age, race, BMI, body weight, mutation status in IL36RN, concurrent plaque psoriasis, GPPPGA total score at baseline, and irrespective of any systemic GPP treatment at randomization.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
SPEVIGO (spesolimab-sbzo) injection is a sterile, preservative-free, colorless to slightly brownish-yellow, clear to slightly opalescent solution available as follows:
Storage and Handling
Prefilled syringes
- Store SPEVIGO prefilled syringes refrigerated between 2°C to 8°C (36°F to 46°F) in original carton to protect from light. Do not freeze. Do not use if frozen even if it has been thawed.
Single-dose vials
- Store SPEVIGO vials refrigerated between 2°C to 8°C (36°F to 46°F) in original carton to protect from light. Do not freeze.
- Prior to use, may store unopened SPEVIGO vials at room temperature, 20°C to 25°C (68°F to 77°F), for up to 24 hours in the original carton to protect from light.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Infections
Inform patients that SPEVIGO may lower the ability of their immune system to fight infections. Instruct patients of the importance of communicating any history of infections to the healthcare provider and contacting their healthcare provider if they develop any signs or symptoms of clinically important infection during or after treatment with SPEVIGO [see Warnings and Precautions (5.1)].
Hypersensitivity and Infusion-Related Reactions
Inform patients that hypersensitivity and infusion-related reactions may occur with SPEVIGO. Advise patients to seek immediate medical attention if they experience any symptoms of a serious hypersensitivity reaction [see Warnings and Precautions (5.3)].
Vaccinations
Advise patients to avoid receiving live vaccines during and for at least 16 weeks after treatment with SPEVIGO. Instruct patients to inform the healthcare provider that they are taking SPEVIGO prior to a potential vaccination [see Warnings and Precautions (5.4)].
Administration Instructions for Subcutaneous SPEVIGO for Treatment of GPP When Not Experiencing a Flare
If a patient 12 years of age and older or caregiver is to administer subcutaneous SPEVIGO, instruct them on injection technique and assess their ability to inject subcutaneously to ensure the proper administration of SPEVIGO. Provide training to patients or caregivers on preparation and administration of subcutaneous SPEVIGO and on proper needle and syringe disposal [see Dosage and Administration (2.5) and Instructions for Use].
Instruct patients or caregivers to subcutaneously administer two 150 mg/mL prefilled syringes to achieve a complete 300 mg dose of SPEVIGO [see Dosage and Administration (2.5) and Instructions for Use].
-
SPL UNCLASSIFIED SECTION
Manufactured by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
US License Number 2006Licensed from:
Boehringer Ingelheim International GmbH, Ingelheim, GermanySPEVIGO is a registered trademark of and used under license from Boehringer Ingelheim International GmbH.
Copyright © 2024 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED
COL9614BC152024
SPL9616B -
MEDICATION GUIDE
MEDICATION GUIDE
SPEVIGO® (spea VEE go)
(spesolimab-sbzo)
injection, for subcutaneous or intravenous useThis Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 03/2024 What is the most important information I should know about SPEVIGO?
SPEVIGO may cause serious side effects, including:- Infections. SPEVIGO may lower the ability of your or your child's immune system to fight infections and may increase your or your child's risk of infections. Your healthcare provider should check you or your child for infections and tuberculosis (TB) before starting treatment with SPEVIGO and may treat you or your child for TB before you begin treatment with SPEVIGO if you have a history of TB or have active TB. Your healthcare provider should watch you or your child closely for signs and symptoms of TB during or after treatment with SPEVIGO. Tell your healthcare provider right away if you or your child have an infection or have symptoms of an infection during or after treatment with SPEVIGO, including:
- fevers, chills, or sweats
- muscle aches
- cough
- shortness of breath
- blood in your phlegm (mucus)
- burning when you urinate
- urinating more often than normal
-
Allergic reactions and infusion-related reactions. Serious allergic reactions may happen during or after your or your child's SPEVIGO injection. If you or your child have a serious allergic reaction, your healthcare provider will stop treatment with SPEVIGO. If you or your child are given SPEVIGO in a vein (intravenously) and have an infusion-related reaction, your healthcare provider will stop your or your child's SPEVIGO infusion and treat your or your child's symptoms and may restart SPEVIGO at a slower infusion rate.
Tell your healthcare provider or get emergency medical help right away if you or your child get any of the following symptoms during or after your or your child's SPEVIGO injection:
- feeling faint, dizzy, or lightheaded
- swelling of your face, eyelids, lips, mouth, tongue, or throat
- trouble breathing or throat tightness
- fever
- mouth sores
- chest tightness
- hives or skin rash that is different than the rash from generalized pustular psoriasis (GPP)
- itching
- swollen lymph nodes
See "What are the possible side effects of SPEVIGO?" for more information about side effects. What is SPEVIGO?
SPEVIGO is a prescription medicine used to treat generalized pustular psoriasis (GPP) in adults and children 12 years of age and older who weigh at least 88 pounds (40 kg).
It is not known if SPEVIGO is safe and effective in children under 12 years of age or who weigh less than 88 pounds (40 kg).Do not receive SPEVIGO if you or your child have had a severe or life-threatening allergic reaction to spesolimab-sbzo or any of the ingredients in SPEVIGO. See the end of this Medication Guide for a complete list of ingredients in SPEVIGO. Before you or your child receive SPEVIGO, tell your healthcare provider about all of your medical conditions, including if you or your child: - have an infection that does not go away or that keeps coming back. See "What is the most important information I should know about SPEVIGO?"
- have TB or have been in close contact with someone with TB.
- have recently received or are scheduled to receive an immunization (vaccine). You or your child should not receive live vaccines during and for at least 16 weeks after treatment with SPEVIGO. You or your child should be brought up to date with all vaccines before starting SPEVIGO.
- are pregnant or plan to become pregnant. It is not known if SPEVIGO can harm your or your child's unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if SPEVIGO passes into your breast milk. Talk to your healthcare provider about the best way to feed your or your child's baby during treatment with SPEVIGO.
How will I receive SPEVIGO?
When given under the skin (subcutaneously) for treatment of GPP when not experiencing a flare:- Read the detailed "Instructions for Use" that comes with SPEVIGO for information on how to prepare and inject a dose of SPEVIGO and how to properly throw away (dispose of) used SPEVIGO prefilled syringes.
- Your healthcare provider will tell you how much SPEVIGO to inject and when to inject it. Use SPEVIGO exactly as your healthcare provider tells you to use it.
- If your healthcare provider decides that you or a caregiver can give your or your child's injections of SPEVIGO at home, you should receive training on the right way to prepare and inject SPEVIGO. Do not try to inject SPEVIGO until you have been shown the right way by your healthcare provider. In children 12 to 17 years of age, SPEVIGO should be given under supervision of an adult.
- If you miss a dose of SPEVIGO, inject your or your child's dose as soon as possible. Then, inject your or your child's next dose at the regular scheduled time. In case you are not sure when to inject SPEVIGO, call your healthcare provider.
- Your healthcare provider will give you or your child SPEVIGO through a needle placed in your vein (intravenous infusion) over 90 minutes.
- SPEVIGO is usually given one time. If GPP flare symptoms continue, your healthcare provider will decide if you should receive an additional treatment with SPEVIGO after 1 week.
What are the possible side effects of SPEVIGO?
SPEVIGO may cause serious side effects. See "What is the most important information I should know about SPEVIGO?"
The most common side effects of SPEVIGO for GPP flare treatment include:- feeling tired or weak
- nausea and vomiting
- headache
- itching or itchy bumps
- a collection of blood under the skin at the infusion site or bruising
- urinary tract infection
The most common side effects of SPEVIGO for treatment of GPP when not experiencing a flare include: - redness, pain, swelling, hardening, hives, or warmth at the injection site
- joint pain
- urinary tract infection
- itching
These are not all of the possible side effects of SPEVIGO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store SPEVIGO? - Store SPEVIGO prefilled syringes in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not freeze SPEVIGO. Do not use SPEVIGO if frozen, even if it has been thawed.
- Store SPEVIGO in the original carton until use to protect from light.
General information about the safe and effective use of SPEVIGO.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use SPEVIGO for a condition for which it was not prescribed. Do not give SPEVIGO to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about SPEVIGO that is written for health professionals.What are the ingredients in SPEVIGO?
Active ingredient: spesolimab-sbzo
Inactive ingredients: arginine hydrochloride, glacial acetic acid, polysorbate 20, sodium acetate, sucrose, and Water for InjectionManufactured by: Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT 06877 USA
US License Number 2006
Licensed from: Boehringer Ingelheim International GmbH, Ingelheim, Germany
SPEVIGO is a registered trademark of and used under license from Boehringer Ingelheim International GmbH.
Copyright © 2024 Boehringer Ingelheim International GmbH ALL RIGHTS RESERVED
COL9614BC152024
For more information about SPEVIGO, including current prescribing information and Medication Guide, go to www.SPEVIGO.com, scan the code below, or call Boehringer Ingelheim Pharmaceuticals, Inc. at 1-800-542-6257.
-
INSTRUCTIONS FOR USESPEVIGO® (spea VEE go)(spesolimab-sbzo) injection, for subcutaneous use
This Instructions for Use contains information on how to inject SPEVIGO.
Read both sides of this Instructions for Use before you use SPEVIGO for the first time and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your or your child's medical condition or treatment. Your healthcare provider should show you or your child the right way to inject SPEVIGO before you try to inject yourself or your child for the first time. In children 12 to 17 years of age, SPEVIGO should be given under supervision of an adult.
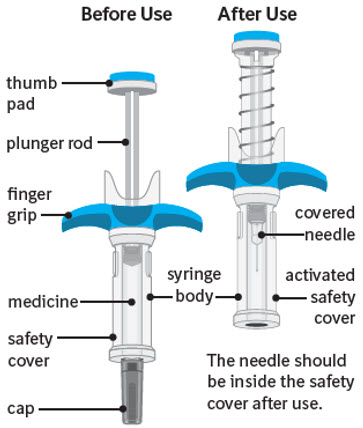
SPEVIGO comes in a prefilled syringe with a safety cover.
SPEVIGO is for one-time use only. Do not reuse the prefilled syringes.
Your healthcare provider has prescribed a dose of SPEVIGO for you or your child that requires 2 injections (2 prefilled syringes) to deliver a complete dose. You must inject the contents of both SPEVIGO prefilled syringes that come in the carton to deliver the complete dose.
Getting to know SPEVIGO:
SPEVIGO comes in a prefilled syringe with a safety cover. The needle is pulled back into the safety cover after injection.
Guide to parts:
The figure below shows SPEVIGO before use, and after use with the activated safety cover.
Important information you need to know before injecting SPEVIGO:
- You must inject the contents of both SPEVIGO prefilled syringes to deliver a complete dose.
- Inspect the SPEVIGO carton to be sure that you have the correct medicine, 2 prefilled syringes for your or your child's prescribed dose, for any damage, and the expiration date.
- Do not use SPEVIGO if the liquid is cloudy or contains flakes or large or colored particles.
- Do not use SPEVIGO if the expiration date (EXP) has passed.
- Do not use SPEVIGO if the prefilled syringe has been dropped.
- Do not remove the cap until you are ready to inject.
- Inject SPEVIGO under the skin (subcutaneous injection) in either the upper thighs or stomach-area (abdomen). Do not inject SPEVIGO into any other area of the body.
Storing SPEVIGO:
- Store SPEVIGO prefilled syringes in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not freeze SPEVIGO. Do not use SPEVIGO if frozen, even if it has been thawed.
- Store SPEVIGO in the original carton until use to protect from light.
Keep SPEVIGO and all medicines out of the reach of children.
Step 1 Gather supplies 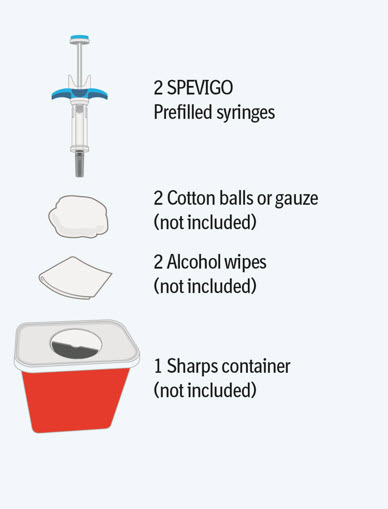
- Take the SPEVIGO carton out of the refrigerator and remove the prefilled syringes from the carton.
- Gather supplies listed above and place them on a clean, flat work surface in a well-lit area.
- If you do not have all the supplies listed above, contact your pharmacist.
- See Step 10: "Disposing of the used SPEVIGO prefilled syringes and caps."
Step 2 Preparing to inject SPEVIGO 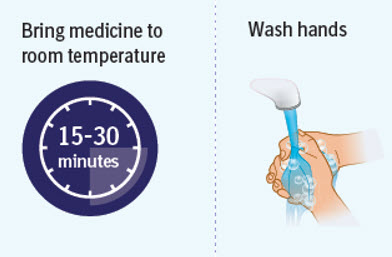
- Wait 15 to 30 minutes to allow the medicine to reach room temperature to avoid discomfort during injection. Do not speed up the warming process in any way, such as using the microwave or placing the syringe in warm water.
- Do not leave the prefilled syringes in direct sunlight.
- Do not remove the needle cap until you are ready to inject.
- Wash your hands well with soap and water and dry them.
Step 3 Inspecting the prefilled syringes 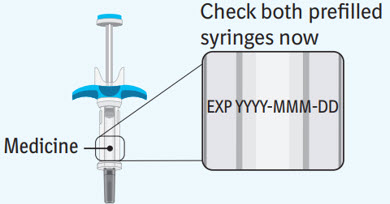
Check both prefilled syringes now: - Check to make sure the medicine name SPEVIGO and dose on your prefilled syringes match your or your child's prescribed dose.
- Check the expiration date on both prefilled syringes. Do not use if the expiration date has passed.
- Check both prefilled syringes for damage, cracks, and leakage. Do not use if any part of the prefilled syringes appear cracked, broken, or are leaking.
- Make sure the medicine in both prefilled syringes is clear and colorless to slightly brownish-yellow. It may contain tiny white or clear particles. Do not use if the medicine is cloudy or has flakes or large or colored particles in it.
- It is normal to see air bubbles, they do not need to be removed.
- Do not use if the SPEVIGO prefilled syringes have been dropped.
Preparing for your first injection 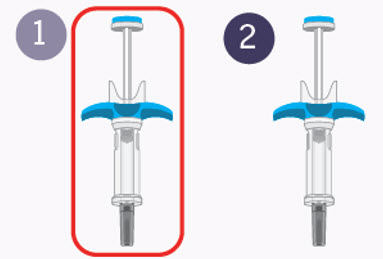
Prepare for the first of 2 injections.
You will repeat the following steps with the second syringe right away after your first injection.
2 injections are needed for a complete dose.Step 4 Choosing the injection site 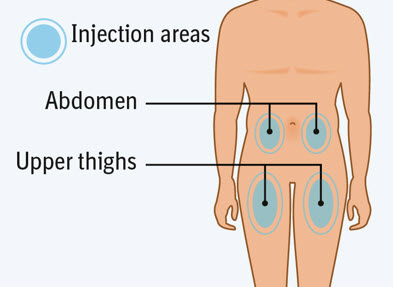
Choose an injection site. - You may use an area on your:
- upper thighs or
- stomach-area (abdomen), except for an area 2 inches around your navel (belly button).
- Choose a different injection site each time you inject, at least 1 inch away from the last injection site. Alternate between upper thigh or stomach-area for each complete dose.
- Do not inject an area near your waistline or belly button.
- Do not inject into areas that are tender, bruised, red, hard, or scarred.
- Do not inject through clothes.
Step 5 Clean the injection site 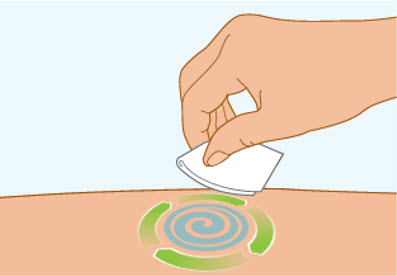
- Clean the injection site with an alcohol wipe and let air dry.
- Do not touch this area again before injecting.
- Do not fan or blow on the clean area.

Step 6 Removing the cap from the prefilled syringe 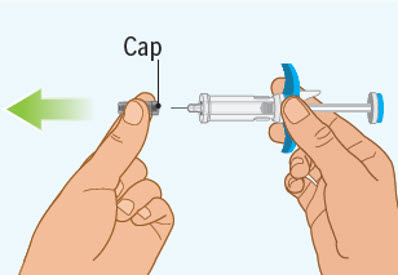
- Hold the prefilled syringe by the finger grip with one hand. With the other hand, pull the cap straight off.
- Do not pull on or hold the plunger rod.
- Do not twist the cap. Twisting the cap could damage the needle.
- Do not use the prefilled syringe if the needle is bent or damaged. If you accidentally bend the needle, do not attempt to straighten it.
- Throw away the cap. See Step 10: "Disposing of the used SPEVIGO prefilled syringes and caps.
- Inject SPEVIGO right away after removing the cap.
- Do not try to recap the needle. Re-capping can lead to needle-stick injury.
- Do not touch the needle or let the needle touch anything before injecting.
Step 7 Pinch the skin 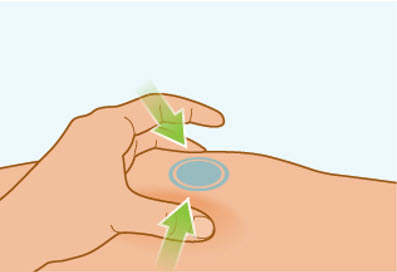
- Gently pinch the area of cleaned skin around your injection site and hold it firmly.
- Keep the skin pinched during the entire injection. You will inject into the pinched skin.
- Do not let go until you have removed the needle from your skin at end of the injection.
Step 8 Injecting SPEVIGO 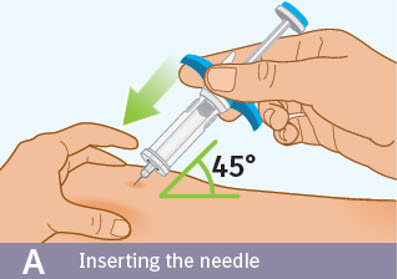
Inserting the needle: - Hold the prefilled syringe by the blue finger grip. Avoid touching the blue thumb pad.
- Using a quick, "dart-like" motion, insert the needle into the pinched skin at about a 45-degree angle.
- Do not move the needle while inserting or during the injection.
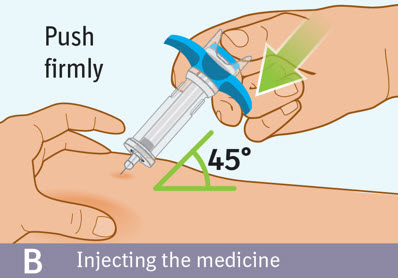
Injecting the medicine: - Use your thumb to slowly press down on the blue thumb pad to push the plunger rod down inside the syringe body.
- Continue pressing on the blue thumb pad until the plunger rod has moved all the way down.
- Make sure that the blue thumb pad cannot be pressed any further so that the built-in safety cover can be activated.
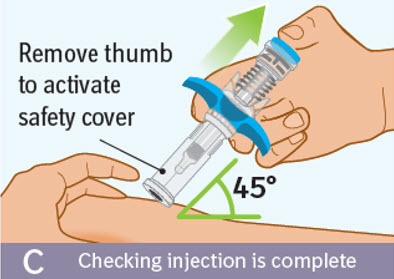
Checking injection is complete: -
Slowly remove your thumb from the blue thumb pad, to move the needle out of your skin and up into the safety cover.
- Check that the thumb pad springs back and that the needle is inside the safety cover.
- If the needle is not inside the safety cover call your healthcare provider. You may not have received a full dose.
- If there is bleeding, press a cotton ball or gauze on the site for a few seconds.
- Do not rub the injection site.
- Apply an adhesive bandage if needed.
Step 9 Second injection 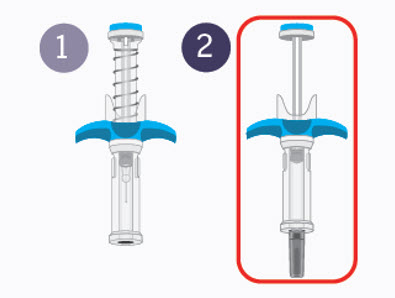
-
Choose a different injection site.
The new injection site should be at least 1 inch away from last injection site. Alternate between upper thigh or stomach-area for each complete dose. - Get second prefilled syringe.
- Repeat steps 4 through 8 right away.
- Then continue to step 10.
You must inject the contents of both SPEVIGO prefilled syringes to give a complete dose.Step 10 Disposing of the used SPEVIGO prefilled syringes and caps 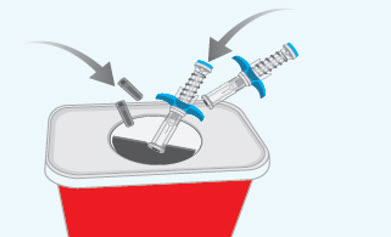
- Put the used prefilled syringes and caps in an FDA-cleared sharps disposal container right away after use.
- Do not throw away (dispose of) the prefilled syringes and caps in the household trash.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- Made of a heavy-duty plastic,
- Can be closed with a tight fitting, puncture-resistant lid, without sharps being able to come out,
- Upright and stable during use,
- Leak resistant, and
- Properly labeled to warn of hazardous waste inside the container.
- Do not reuse the prefilled syringes.
- Do not throw away (dispose of) your used sharps disposal container in your household trash unless your community guidelines permit this.
- Do not recycle your used sharps disposal container.
If you have any problems with your injection, do not use another SPEVIGO prefilled syringe. Call your healthcare provider or pharmacist for help.
For more information call 1-800-542-6257.Manufactured by:
Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT 06877 USA
US License Number 2006Licensed from: Boehringer Ingelheim International GmbH, Ingelheim, Germany
SPEVIGO is a registered trademark of and used under license from Boehringer Ingelheim International GmbH.Copyright © 2024 Boehringer Ingelheim International GmbH ALL RIGHTS RESERVED
COL12534AC152024For more information about SPEVIGO, including current prescribing information and Medication Guide, go to www.SPEVIGO.com, scan the code below, or call Boehringer Ingelheim Pharmaceuticals, Inc. at 1-800-542-6257.

This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Approved: 03/2024 -
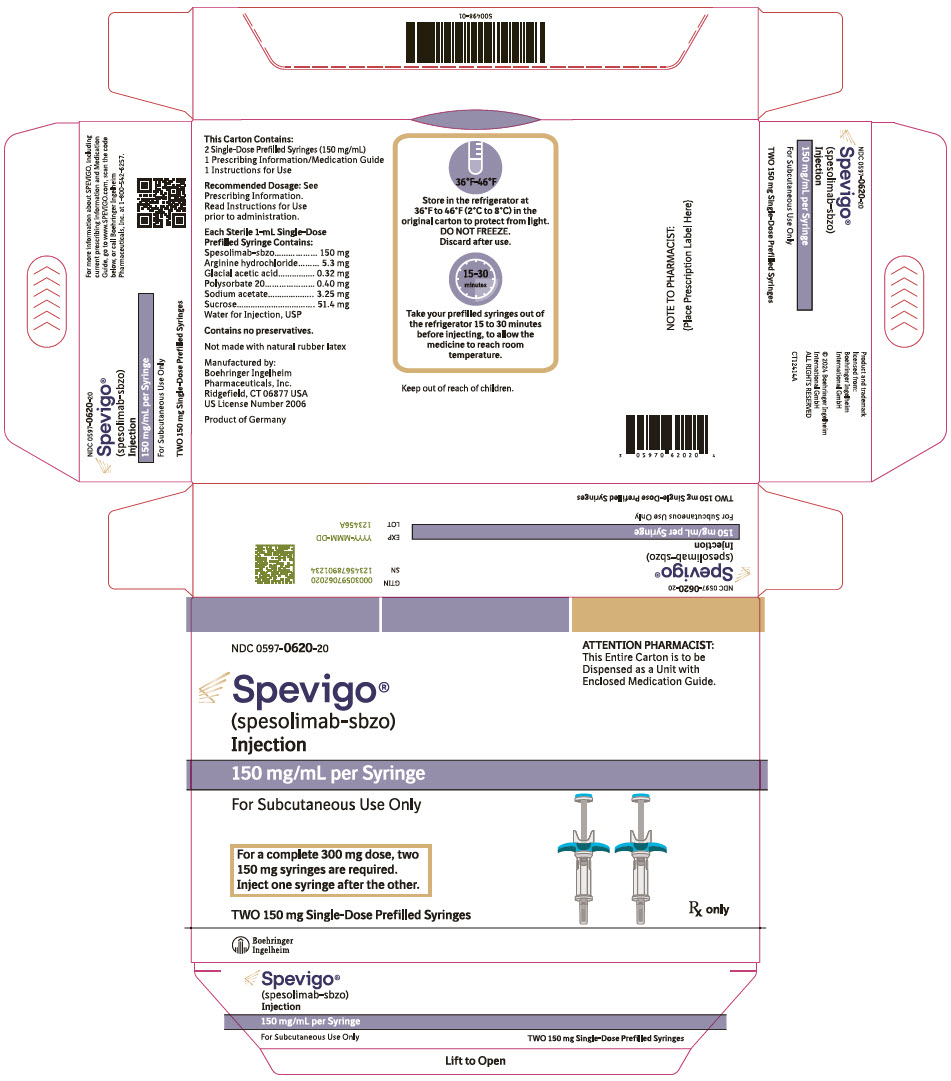
PRINCIPAL DISPLAY PANEL - 150 mg Syringe Carton
NDC 0597-0620-20
ATTENTION PHARMACIST:
This Entire Carton is to be
Dispensed as a Unit with
Enclosed Medication Guide.Spevigo®
(spesolimab-sbzo)
Injection150 mg/mL per Syringe
For Subcutaneous Use Only
For a complete 300 mg dose, two
150 mg syringes are required.
Inject one syringe after the other.TWO 150 mg Single-Dose Prefilled Syringes
Rx only
Boehringer
Ingelheim
-
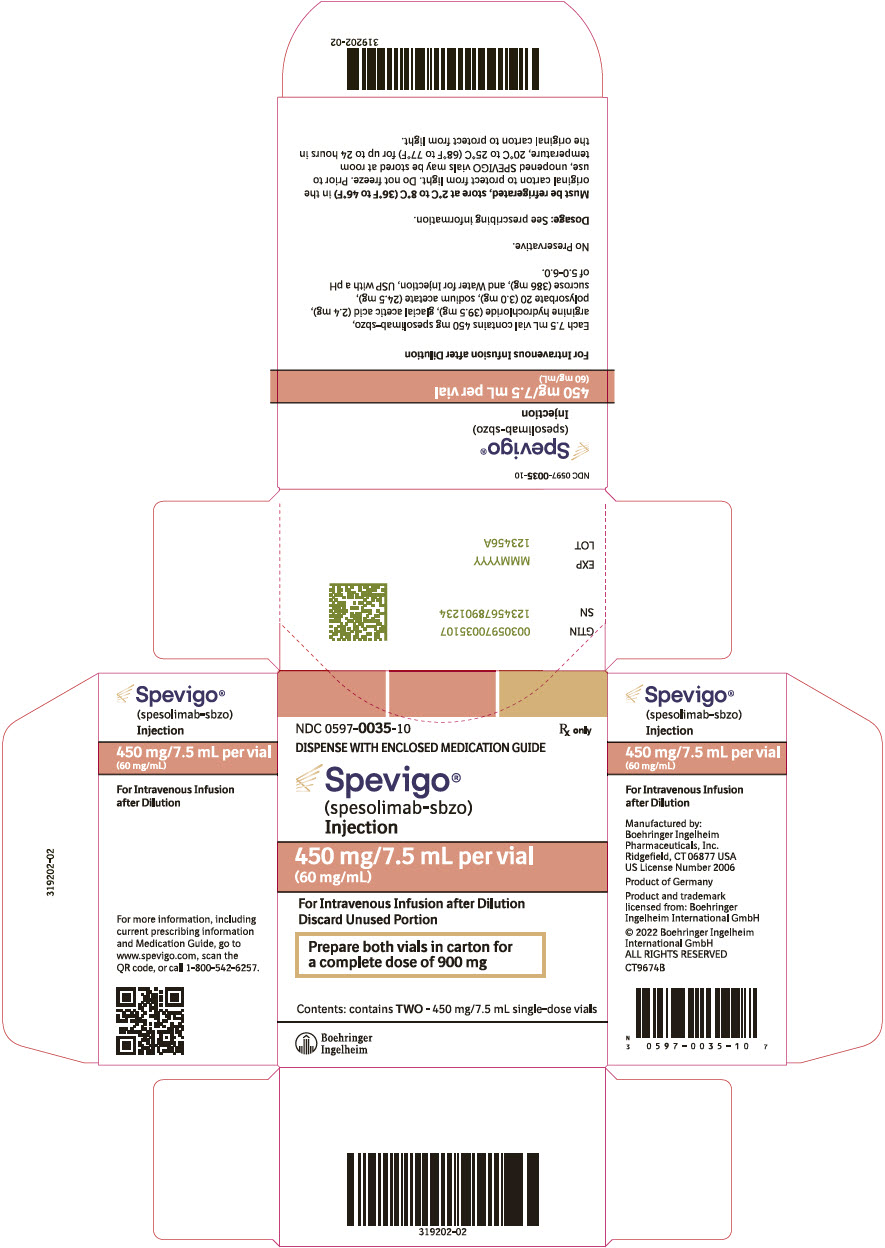
PRINCIPAL DISPLAY PANEL - 450 mg/7.5 mL Vial Carton
NDC 0597-0035-10
Rx onlyDISPENSE WITH ENCLOSED MEDICATION GUIDE
Spevigo®
(spesolimab-sbzo)
Injection450 mg/7.5 mL per vial
(60 mg/mL)For Intravenous Infusion after Dilution
Discard Unused PortionPrepare both vials in carton for
a complete dose of 900 mgContents: contains TWO - 450 mg/7.5 mL single-dose vials
Boehringer
Ingelheim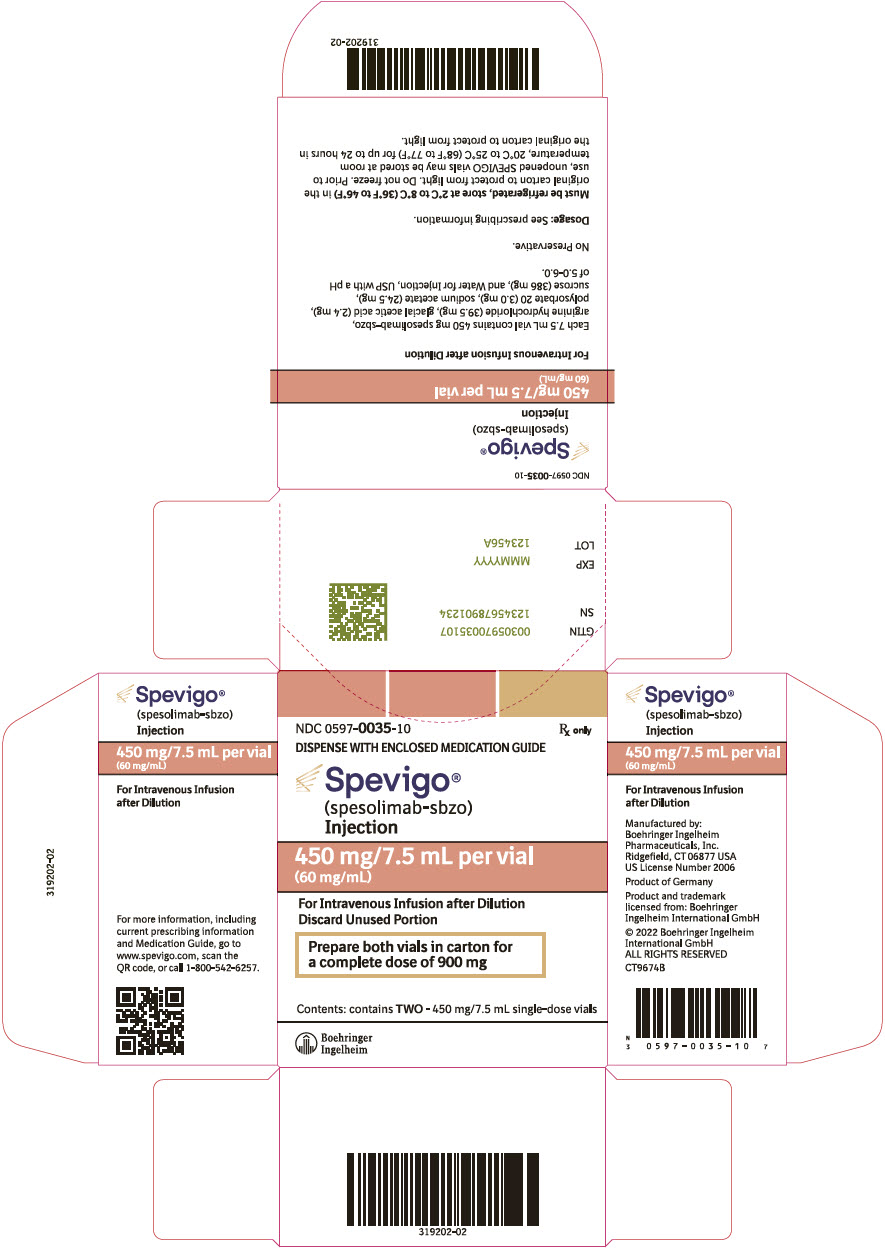
-
INGREDIENTS AND APPEARANCE
SPEVIGO
spesolimab-sbzo injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0597-0035 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SPESOLIMAB (UNII: 5IB2J79MCX) (SPESOLIMAB - UNII:5IB2J79MCX) SPESOLIMAB 60 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0597-0035-10 2 in 1 CARTON 09/01/2022 1 7.5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761244 09/01/2022 SPEVIGO
spesolimab-sbzo injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0597-0620 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SPESOLIMAB (UNII: 5IB2J79MCX) (SPESOLIMAB - UNII:5IB2J79MCX) SPESOLIMAB 150 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0597-0620-20 2 in 1 CARTON 03/18/2024 1 NDC:0597-0620-10 150 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761244 03/18/2024 Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) Registrant - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) Establishment Name Address ID/FEI Business Operations Boehringer Ingelheim Pharma GmbH and Co. KG 340700520 MANUFACTURE(0597-0035, 0597-0620) , API MANUFACTURE(0597-0035, 0597-0620) , PACK(0597-0035, 0597-0620) , LABEL(0597-0035, 0597-0620) Establishment Name Address ID/FEI Business Operations West-Ward Columbus Inc. 058839929 PACK(0597-0035) , LABEL(0597-0035)