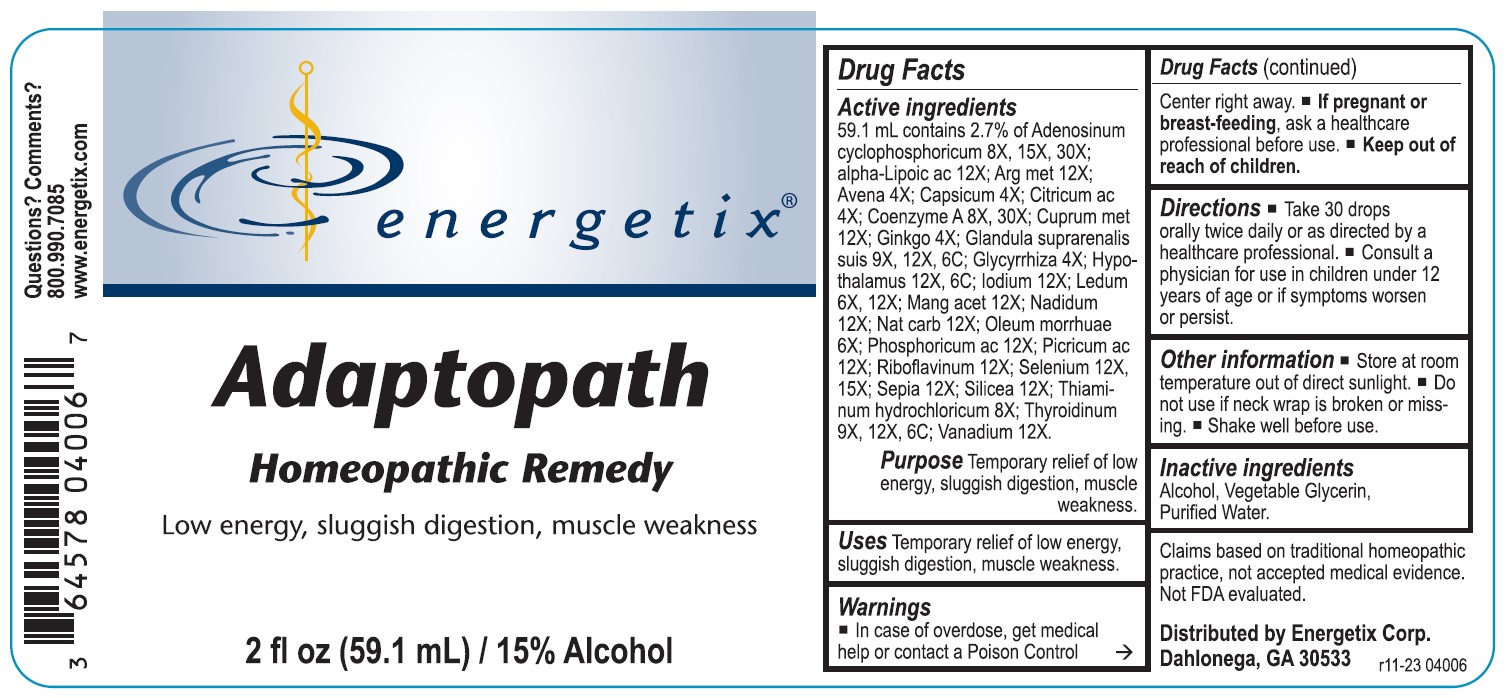
Label: ADAPTOPATH (adenosinum cyclophosphoricum, alpha-lipoicum acidum, argentum metallicum, avena sativa, capsicum annuum, citricum acidum, coenzyme a, cuprum metallicum, ginkgo biloba, glandula suprarenalis suis, glycyrrhiza glabra, hypothalamus (bovine), iodium, ledum palustre, manganum aceticum, nadidum, natrum carbonicum, oleum morrhuae, phosphoricum acidum, picricum acidum, riboflavinum, selenium metallicum sepia, silicea, thiaminum hydrochloricum, thyroidinum- bovine, vanadium metallicum liquid
- NDC Code(s): 64578-0095-1
- Packager: Energetix Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active ingredients 59.1 mL contains 2.7% of Adenosinum cyclophosphoricum 8X, 15X, 30X; alpha-Lipoicum ac 12; Arg met 12X; Avena 4X; Capsicum 4X; Citricum ac 4X; Coenzyme A 8X, 30X; Cuprum met 12X; Ginkgo 4X; Glandula suprarenalis suis 9X, 12X, 6C; Glycyrrhiza 4X; Hypothalamus 12X, 6C; Iodium 12X; Ledum 6X, 12X; Mang acet 12X; Nadidum 12X; Nat carb 12X; Oleum morrhuae 6X; Phosphoricum ac 12X; Picricum ac 12X; Riboflavinum 12X; Selenium 12X, 15X; Sepia 12X; Silicea 12X; Thiaminum hydrochloricum 8X; Thyroidinum 9X, 12X, 6C; Vanadium 12X.
Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
- WARNINGS
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ADAPTOPATH
adenosinum cyclophosphoricum, alpha-lipoicum acidum, argentum metallicum, avena sativa, capsicum annuum, citricum acidum, coenzyme a, cuprum metallicum, ginkgo biloba, glandula suprarenalis suis, glycyrrhiza glabra, hypothalamus (bovine), iodium, ledum palustre, manganum aceticum, nadidum, natrum carbonicum, oleum morrhuae, phosphoricum acidum, picricum acidum, riboflavinum, selenium metallicum sepia, silicea, thiaminum hydrochloricum, thyroidinum (bovine), vanadium metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64578-0095 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ADENOSINE CYCLIC PHOSPHATE (UNII: E0399OZS9N) (ADENOSINE CYCLIC PHOSPHATE - UNII:E0399OZS9N) ADENOSINE CYCLIC PHOSPHATE 8 [hp_X] in 1 mL .ALPHA.-LIPOIC ACID (UNII: 73Y7P0K73Y) (.ALPHA.-LIPOIC ACID - UNII:73Y7P0K73Y) .ALPHA.-LIPOIC ACID 12 [hp_X] in 1 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 1 mL AVENA SATIVA FLOWERING TOP (UNII: MA9CQJ3F7F) (AVENA SATIVA FLOWERING TOP - UNII:MA9CQJ3F7F) AVENA SATIVA FLOWERING TOP 4 [hp_X] in 1 mL CAPSICUM (UNII: 00UK7646FG) (CAPSICUM - UNII:00UK7646FG) CAPSICUM 4 [hp_X] in 1 mL ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 4 [hp_X] in 1 mL COENZYME A (UNII: SAA04E81UX) (COENZYME A - UNII:SAA04E81UX) COENZYME A 8 [hp_X] in 1 mL COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 12 [hp_X] in 1 mL GINKGO (UNII: 19FUJ2C58T) (GINKGO - UNII:19FUJ2C58T) GINKGO 4 [hp_X] in 1 mL SUS SCROFA ADRENAL GLAND (UNII: 398IYQ16YV) (SUS SCROFA ADRENAL GLAND - UNII:398IYQ16YV) SUS SCROFA ADRENAL GLAND 9 [hp_X] in 1 mL GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) (GLYCYRRHIZA GLABRA - UNII:2788Z9758H) GLYCYRRHIZA GLABRA 4 [hp_X] in 1 mL BOS TAURUS HYPOTHALAMUS (UNII: S6G2NLH4Y7) (BOS TAURUS HYPOTHALAMUS - UNII:S6G2NLH4Y7) BOS TAURUS HYPOTHALAMUS 12 [hp_X] in 1 mL IODINE (UNII: 9679TC07X4) (IODINE - UNII:9679TC07X4) IODINE 12 [hp_X] in 1 mL LEDUM PALUSTRE TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) LEDUM PALUSTRE TWIG 6 [hp_X] in 1 mL MANGANESE ACETATE TETRAHYDRATE (UNII: 9TO51D176N) (MANGANESE CATION (2+) - UNII:H6EP7W5457) MANGANESE ACETATE TETRAHYDRATE 12 [hp_X] in 1 mL NADIDE (UNII: 0U46U6E8UK) (NADIDE - UNII:0U46U6E8UK) NADIDE 12 [hp_X] in 1 mL SODIUM CARBONATE (UNII: 45P3261C7T) (CARBONATE ION - UNII:7UJQ5OPE7D) SODIUM CARBONATE 12 [hp_X] in 1 mL COD LIVER OIL (UNII: BBL281NWFG) (COD LIVER OIL - UNII:BBL281NWFG) COD LIVER OIL 6 [hp_X] in 1 mL PHOSPHORIC ACID (UNII: E4GA8884NN) (PHOSPHORIC ACID - UNII:E4GA8884NN) PHOSPHORIC ACID 12 [hp_X] in 1 mL PICRIC ACID (UNII: A49OS0F91S) (PICRIC ACID - UNII:A49OS0F91S) PICRIC ACID 12 [hp_X] in 1 mL RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 12 [hp_X] in 1 mL SELENIUM (UNII: H6241UJ22B) (SELENIUM - UNII:H6241UJ22B) SELENIUM 12 [hp_X] in 1 mL SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 12 [hp_X] in 1 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 12 [hp_X] in 1 mL THIAMINE HYDROCHLORIDE (UNII: M572600E5P) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE HYDROCHLORIDE 8 [hp_X] in 1 mL THYROID, BOVINE (UNII: MN18OTN73W) (THYROID, BOVINE - UNII:MN18OTN73W) THYROID, BOVINE 9 [hp_X] in 1 mL VANADIUM (UNII: 00J9J9XKDE) (VANADIUM - UNII:00J9J9XKDE) VANADIUM 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64578-0095-1 59.1 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 05/23/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/22/2014 Labeler - Energetix Corporation (969572502)