Label: MIRTAZAPINE tablet, film coated
- NDC Code(s): 63739-098-10, 63739-099-10
- Packager: McKesson Corporation dba SKY Packaging
- This is a repackaged label.
- Source NDC Code(s): 60505-0247, 60505-0248
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MIRTAZAPINE TABLETS
safely and effectively. See full prescribing information for MIRTAZAPINE TABLETS.
MIRTAZAPINE TABLETS, for oral use
Initial U.S. Approval: 1996WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
See full prescribing information for complete boxed warning.
Increased risk of suicidal thoughts and behavior in pediatric and young adult patients
taking antidepressants. Closely monitor all antidepressant-treated patients for clinical
worsening and emergence of suicidal thoughts and behaviors. Mirtazapine tablets is not
approved for use in pediatric patients. (5.1, 8.4)INDICATIONS AND USAGE
Mirtazapine Tablets is indicated for the treatment of major depressive disorder (MDD) in adults. (1) (1)
DOSAGE AND ADMINISTRATION
• Starting dose: 15-mg once daily; may increase up to maximum recommended dose of 45 mg
once daily. (2.1)
• Administer orally once daily, preferably in the evening prior to sleep. (2.1)
• Reduce dose gradually when discontinuing mirtazapine tablets. (2.6, 5.14) (2)DOSAGE FORMS AND STRENGTHS
• Tablets: 15 mg scored and 30 mg scored. (3) (3)
CONTRAINDICATIONS
• Concomitant use of monoamine oxidase inhibitors (MAOIs) or use within 14 days of stopping
MAOIs. (2.4, 4,7)
• Known hypersensitivity to mirtazapine or any of the excipients in mirtazapine tablets. (4) (4)WARNINGS AND PRECAUTIONS
• Agranulocytosis: If sore throat, fever, stomatitis or signs of infection occur, along with a low
white blood cell count, treatment with mirtazapine tablets should be discontinued and the
patient should be closely monitored. (5.2)
• Serotonin Syndrome: Increased risk when co-administered with other serotonergic drugs (e.g.,
SSRI, SNRI, triptans), but also when taken alone. If it occurs, discontinue mirtazapine tablets and
initiate supportive treatment. (2.4, 4, 5.3, 7)
• Angle-Closure Glaucoma: Angle closure glaucoma has occurred in patients with untreated
anatomically narrow angles treated with antidepressants. (5.4) (5)• QT Prolongation: Use mirtazapine tablets with caution in patients with risk factors for QT
prolongation. (5.5, 7) (5)• Drug Reaction with Eosinophilia and System Symptoms (DRESS): Discontinue Mirtazapine
tablets if DRESS is suspected. (5.6) (5)• Increased Appetite/Weight Gain: Mirtazapine tablets have been associated with increased
appetite and weight gain. (5.7)
• Somnolence: May impair judgment, thinking and/or motor skills. Use with caution when
engaging in activities requiring alertness, such as driving or operating machinery. (5.8, 7)
• Activation of Mania/Hypomania: Screen patients for bipolar disorder prior to initiating treatment.
(2.3, 5.9)
• Seizures: Use with caution in patients with a seizure disorder. (5.10)
• Elevated Cholesterol/Triglycerides: Has been reported with mirtazapine tablet use. (5.11)
• Hyponatremia: May occur as a result of treatment with serotonergic antidepressants, including
mirtazapine tablets. (5.12)
• Transaminase Elevations: Clinically significant elevations have occurred. Use with caution in
patients with impaired hepatic function. (5.13) (5)ADVERSE REACTIONS
Most common adverse reactions ((≥5% or greater and twice placebo) were somnolence, increased
appetite, weight gain, and dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Apotex Corp. at, at 1-800-706-5575 or
FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)DRUG INTERACTIONS
• Strong CYP3A inducers: Dosage increase may be needed for mirtazapine tablets with
concomitant use of strong CYP3A inducers. (2.5, 7)
• Strong CYP3A inhibitors: Dosage decrease may be needed when mirtazapine tablets are
coadministered with strong CYP3A inhibitors. (2.5, 7)
• Cimetidine: Dosage decrease may be needed when mirtazapine tablets are coadministered with
cimetidine. (2.5, 7)
• Warfarin: Monitor INR during concomitant use. (7) (7)USE IN SPECIFIC POPULATIONS
• Geriatric Use: Use with caution in elderly patients. (5.12, 5.15, 8.5)
• Renal impairment: Dosage decrease may be needed in patients with moderate to severe renal
impairment. (8.6)
• Hepatic impairment: Dosage decrease may be needed in patients with hepatic impairment. (8.6) (8)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide (8)
Revised: 02/2024 (8)See 17 for Medication Guide.
Revised: 3/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.3 Screen for Bipolar Disorder Prior to Starting Mirtazapine Tablets
2.4 Switching Patients to or from a Monoamine Oxidase Inhibitor Antidepressant
2.5 Dosage Modifications Due to Drug Interactions
2.6 Discontinuation of Mirtazapine Tablets Treatment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
5.2 Agranulocytosis
5.3 Serotonin Syndrome
5.4 Angle-closure Glaucoma
5.5 QT Prolongation and Torsades de Pointes
5.6 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
5.7 Increased Appetite and Weight Gain
5.8 Somnolence
5.9 Activation of Mania or Hypomania
5.10 Seizures
5.11 Elevated Cholesterol and Triglycerides
5.12 Hyponatremia
5.13 Transaminase Elevations
5.14 Discontinuation Syndrome
5.15 Use in Patients with Concomitant Illness
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
ECG Changes
Other Adverse Events Observed During the Premarketing Evaluation of Mirtazapine Tablets
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal or Hepatic Impairment
10 OVERDOSAGE
Human Experience
Overdose Management
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- BOXED WARNING (What is this?)
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended starting dose for mirtazapine tablets is 15 mg once daily, administered orally,
preferably in the evening prior to sleep. If patients do not have an adequate response to the initial
15 mg dose, increase the dose up to a maximum of 45 mg per day. Dose changes should not be
made in intervals of less than 1 to 2 weeks to allow sufficient time for evaluation of response to a
given dose [see Clinical Pharmacology (12.3)].2.3 Screen for Bipolar Disorder Prior to Starting Mirtazapine Tablets
Prior to initiating treatment with mirtazapine tablets or another antidepressant, screen patients for a
personal or family history of bipolar disorder, mania, or hypomania [see Warnings and Precautions
(5.8)].2.4 Switching Patients to or from a Monoamine Oxidase Inhibitor Antidepressant
At least 14 days must elapse between discontinuation of a monoamine oxidase inhibitor (MAOI)
antidepressant and initiation of mirtazapine tablets. In addition, at least 14 days must elapse after
stopping mirtazapine tablets before starting an MAOI antidepressant [ see Contraindications (4) and
Warnings and Precautions (5.3)].2.5 Dosage Modifications Due to Drug Interactions
Strong CYP3A Inducers
An increase in dosage of mirtazapine tablets may be needed with concomitant strong CYP3A
inducer (e.g., carbamazepine, phenytoin, rifampin) use. Conversely, a decrease in dosage of
mirtazapine tablets may be needed if the CYP3A inducer is discontinued [see Drug Interactions (7)].
Strong CYP3A Inhibitors
A decrease in dosage of mirtazapine tablets may be needed with concomitant use of strong
CYP3A4 inhibitors (e.g., ketoconazole, clarithromycin). Conversely, an increase in dosage of
mirtazapine tablets may be needed if the CYP3A inhibitor is discontinued [see Drug Interactions (7)].
Cimetidine
A decrease in dosage of mirtazapine tablets may be needed with concomitant use of cimetidine.
Conversely, an increase in dosage of mirtazapine tablets may be needed if cimetidine is
discontinued [see Drug Interactions (7)]. -
3 DOSAGE FORMS AND STRENGTHS
Mirtazapine Tablets, USP 15 mg are available for oral administration as pale yellow, oval-shaped,
scored, film-coated tablets imprinted "APO" on one side and "MI" bisect "15" on the other side.
Mirtazapine Tablets, USP 30 mg are available for oral administration as light pink, oval-shaped,
scored, film-coated tablets imprinted "APO" on one side and "MI" bisect "30" on the other side. -
4 CONTRAINDICATIONS
Mirtazapine tablets are contraindicated in patients:
• Taking, or within 14 days of stopping, MAOIs (including the MAOIs linezolid and intravenous
methylene blue) because of an increased risk of serotonin syndrome [see Warnings and
Precautions (5.3), Drug Interactions (7)].
• With a known hypersensitivity to mirtazapine or to any of the excipients in mirtazapine
tablets. Severe skin reactions, including drug reaction with eosinophilia and systemic
symptoms (DRESS), Stevens-Johnson syndrome, bullous dermatitis, erythema multiforme and
toxic epidermal necrolysis have been reported following the use of mirtazapine tablets [see
Warnings and Precautions (5.6), Adverse Reactions (6.2)]. -
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other
antidepressant classes) that included approximately 77,000 adult patients and 4,500 pediatric
patients, the incidence of suicidal thoughts and behaviors in antidepressant-treated patients age 24
years and younger was greater than in placebo-treated patients. There was considerable variation
in risk of suicidal thoughts and behaviors among drugs, but there was an increased risk identified in
young patients for most drugs studied. There were differences in absolute risk of suicidal thoughts
and behaviors across the different indications, with the highest incidence in patients with MDD. The
drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1000
patients treated are provided in Table 1.Table 1: Risk Differences of the Number of Patients with Suicidal Thoughts and Behavior in
the Pooled Placebo-Controlled Trials of Antidepressants in Pediatric and Adult PatientsAge Range Drug-placebo Difference in Number of Patients with Suicidal Thoughts and Behaviour in the Pooled Placebo-Controlled Trials of Antidepressants in Pediatric and Adult Patients Increases Compared to Placebo <18 years old 14 additional patients 18 to 24 years old 5 additional patients Decreases Compared to Placebo 25 to 64 years old 1 fewer patients ≥65 years old 6 fewer patients It is unknown whether the risk of suicidal thoughts and behaviors in children, adolescents, and young
adults extends to longer-term use, i.e., beyond four months. However, there is substantial evidence
from placebo-controlled maintenance trials in adults with MDD that antidepressants delay the
recurrence of depression and that depression itself is a risk factor for suicidal thoughts and behaviors.Monitor all antidepressant-treated patients for any indication of clinical worsening and emergence
of suicidal thoughts and behaviors, especially during the initial few months of drug therapy, and attimes of dosage changes. Counsel family members or caregivers of patients to monitor for changes
in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen,
including possibly discontinuing mirtazapine tablets, in patients whose depression is persistently
worse, or who are experiencing emergent suicidal thoughts or behaviors.5.2 Agranulocytosis
In premarketing clinical trials, 2 (1 with Sjögren’s Syndrome) out of 2796 patients treated with
mirtazapine tablets developed agranulocytosis [absolute neutrophil count (ANC) <500/mm3 with
associated signs and symptoms, e.g., fever, infection, etc.] and a third patient developed severe
neutropenia (ANC <500/mm3 without any associated symptoms). For these 3 patients, onset of
severe neutropenia was detected on days 61, 9, and 14 of treatment, respectively. All 3 patients
recovered after mirtazapine tablets were stopped. If a patient develops a sore throat, fever,
stomatitis, or other signs of infection, along with a low white blood cell (WBC) count, treatment with
mirtazapine tablets should be discontinued and the patient should be closely monitored.5.3 Serotonin Syndrome
Serotonergic antidepressants, including mirtazapine tablets, can precipitate serotonin syndrome, a
potentially life-threatening condition. The risk is increased with concomitant use of other
serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol,
tryptophan, buspirone, amphetamines, and St. John’s Wort) and with drugs that impair metabolism
of serotonin, i.e., MAOIs [see Contraindications (4), Drug Interactions (7)]. Serotonin syndrome can
also occur when these drugs are used alone.
Serotonin syndrome signs and symptoms may include mental status changes (e.g., agitation,
hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure,
dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity,
myoclonus, hyperreflexia, incoordination), seizures, and gastrointestinal symptoms (e.g., nausea,
vomiting, diarrhea).
The concomitant use of mirtazapine tablets with MAOIs is contraindicated. In addition, do not
initiate mirtazapine tablets in a patient being treated with MAOIs such as linezolid or intravenous
methylene blue. No reports involved the administration of methylene blue by other routes (such as
oral tablets or local tissue injection). If it is necessary to initiate treatment with an MAOI such as
linezolid or intravenous methylene blue in a patient taking mirtazapine tablets, discontinue
mirtazapine tablets before initiating treatment with the MAOI [see Contraindications (4), Drug
Interactions (7)].
Monitor all patients taking mirtazapine tablets for the emergence of serotonin syndrome.
Discontinue treatment with mirtazapine tablets and any concomitant serotonergic agents
immediately if the above symptoms occur and initiate supportive symptomatic treatment. If
concomitant use of mirtazapine tablets with other serotonergic drugs is clinically warranted, inform
patients of the increased risk for serotonin syndrome and monitor for symptoms.5.4 Angle-closure Glaucoma
The pupillary dilation that occurs following use of many antidepressant drugs, including mirtazapine
tablets, may trigger an angle-closure attack in a patient with anatomically narrow angles who does
not have a patent iridectomy.5.5 QT Prolongation and Torsades de Pointes
The effect of mirtazapine tablets on QTc interval was assessed in a clinical randomized trial with
placebo and positive (moxifloxacin) controls involving 54 healthy volunteers using exposure
response analysis. This trial showed a positive relationship between mirtazapine concentrations and
prolongation of the QTc interval. However, the degree of QT prolongation observed with both 45 mg
and 75 mg (1.67 times the maximum recommended daily dose) doses of mirtazapine was not at a
level generally considered to be clinically meaningful. During postmarketing use of mirtazapine,
cases of QT prolongation, Torsades de Pointes, ventricular tachycardia, and sudden death, have
been reported [see Adverse Reactions (6.1, 6.2)]. The majority of reports occurred in association
with overdose or in patients with other risk factors for QT prolongation, including concomitant use
of QTc-prolonging medicines [see Drug Interactions (7) and Overdosage (10)]. Exercise caution
when mirtazapine tablets is prescribed in patients with known cardiovascular disease or family
history of QT prolongation, and in concomitant use with other drugs thought to prolong the QTc
interval.5.6 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
Drug reaction with eosinophilia and systemic symptoms (DRESS) has been reported with
postmarketing use of mirtazapine. DRESS may present with a cutaneous reaction (such as rash or
exfoliative dermatitis), eosinophilia, fever, and/or lymphadenopathy with systemic complications
such as hepatitis, nephritis, pneumonitis, myocarditis, and/or pericarditis. DRESS is sometimes
fatal. Discontinue mirtazapine immediately if DRESS is suspected and institute appropriate
treatment [see Contraindications (4), Adverse Reactions (6.2)].5.7 Increased Appetite and Weight Gain
In U.S. controlled clinical studies, appetite increase was reported in 17% of patients treated with
mirtazapine tablets, compared to 2% for placebo. In these same trials, weight gain of ≥7% of body
weight was reported in 7.5% of patients treated with mirtazapine, compared to 0% for placebo. In a
pool of premarketing U.S. clinical studies, including many patients for long-term, open-label
treatment, 8% of patients receiving mirtazapine tablets discontinued for weight gain.
In an 8-week-long pediatric clinical trial of doses between 15 to 45 mg/day, 49% of mirtazapine
tablets-treated pediatric patients had a weight gain of at least 7%, compared to 5.7% of
placebo-treated patients. The safety and effectiveness of mirtazapine tablets in pediatric patients
with MDD have not been established [see Use in Specific Populations (8.4)].5.8 Somnolence
In U.S. controlled studies, somnolence was reported in 54% of patients treated with mirtazapine
tablets, compared to 18% for placebo. In these studies, somnolence resulted in discontinuation for
10.4% of mirtazapine tablets-treated patients, compared to 2.2% for placebo. It is unclear whether
tolerance develops to the somnolent effects of mirtazapine tablets. Because of the potentially
significant effects of mirtazapine tablets on impairment of performance, caution patients about
engaging in activities that require alertness, including operating hazardous machinery and motor
vehicles, until they are reasonably certain that mirtazapine does not affect them adversely. The
concomitant use of benzodiazepines and alcohol with mirtazapine tablets should be avoided [see
Drug Interactions (7)].5.9 Activation of Mania or Hypomania
In patients with bipolar disorder, treating a depressive episode with mirtazapine tablets or another
antidepressant may precipitate a mixed/manic episode. In controlled clinical trials, patients with
bipolar disorder were generally excluded; however, symptoms of mania or hypomania were
reported in 0.2% of patients treated with mirtazapine tablets. Prior to initiating treatment with
mirtazapine tablets, screen patients for any personal or family history of bipolar disorder, mania, or
hypomania.5.10 Seizures
Mirtazapine tablets have not been systematically evaluated in patients with seizure disorders. In
premarketing clinical trials, 1 seizure was reported among the 2796 U.S. and non-U.S. patients
treated with mirtazapine tablets. Mirtazapine tablets should be prescribed with caution in patients
with a seizure disorder.5.11 Elevated Cholesterol and Triglycerides
In U.S. controlled studies, nonfasting cholesterol increases to ≥20% above the upper limits of
normal were observed in 15% of patients treated with mirtazapine tablets, compared to 7% for
placebo. In these same studies, nonfasting triglyceride increases to ≥500 mg/dL were observed in
6% of patients treated with mirtazapine tablets, compared to 3% for placebo.5.12 Hyponatremia
Hyponatremia may occur as a result of treatment with serotonergic antidepressants, including
mirtazapine tablets. Cases with serum sodium lower than 110 mmol/L have been reported.
Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory
impairment, confusion, weakness, and unsteadiness, which may lead to falls. Signs and symptoms
associated with more severe or acute cases have included hallucination, syncope, seizure, coma,
respiratory arrest, and death. In many cases, this hyponatremia appears to be the result of the
syndrome of inappropriate antidiuretic hormone secretion (SIADH).
In patients with symptomatic hyponatremia, discontinue mirtazapine tablets and institute
appropriate medical intervention. Elderly patients, patients taking diuretics, and those who are
volume- depleted may be at greater risk of developing hyponatremia [see Use in Specific
Populations (8.5)].5.13 Transaminase Elevations
Clinically significant ALT (SGPT) elevations ((≥3 times the upper limit of the normal range) were
observed in 2.0% (8/424) of patients treated with mirtazapine tablets in a pool of short-term, U.S.
controlled trials, compared to 0.3% (1/328) of placebo patients. While some patients were
discontinued for the ALT increases, in other cases, the enzyme levels returned to normal despite
continued mirtazapine treatment. Mirtazapine tablets should be used with caution in patients with
impaired hepatic function [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].5.14 Discontinuation Syndrome
There have been reports of adverse reactions upon the discontinuation of mirtazapine tablets
(particularly when abrupt), including but not limited to the following: dizziness, abnormal dreams,
sensory disturbances (including paresthesia and electric shock sensations), agitation, anxiety,
fatigue, confusion, headache, tremor, nausea, vomiting, and sweating, or other symptoms which
may be of clinical significance.
A gradual reduction in the dosage, rather than an abrupt cessation, is recommended [see Dosage
and Administration (2.6)].5.15 Use in Patients with Concomitant Illness
Mirtazapine tablets have not been systematically evaluated or used to any appreciable extent in
patients with a recent history of myocardial infarction or other significant heart disease. Mirtazapine
tablets were associated with significant orthostatic hypotension in early clinical pharmacology trials
with normal volunteers. Orthostatic hypotension was infrequently observed in clinical trials with
depressed patients [see Adverse Reactions (6.1)]. Mirtazapine tablets should be used with caution
in patients with known cardiovascular or cerebrovascular disease that could be exacerbated by
hypotension (history of myocardial infarction, angina, or ischemic stroke) and conditions that would
predispose patients to hypotension (dehydration, hypovolemia, and treatment with antihypertensive
medication). -
6 ADVERSE REACTIONS
The following adverse reactions are described in more detail in other sections of the prescribing
information:
• Hypersensitivity [see Contraindications (4)]
• Suicidal Thoughts and Behaviors [see Warnings and Precautions (5.1)]
• Agranulocytosis [see Warnings and Precautions (5.2)]
• Serotonin Syndrome [see Contraindications (4), Warnings and Precautions (5.3), Drug
Interactions (7)]
• Angle-Closure Glaucoma [see Warnings and Precautions (5.4)]
• QT Prolongation and Torsades de Pointes [see Warnings and Precautions (5.5)]• Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) [see Warnings and
Precautions (5.6)]
• Increased Appetite and Weight Gain [see Warnings and Precautions (5.6)]
• Somnolence [see Warnings and Precautions (5.7)]
• Activation of Mania or Hypomania [see Warnings and Precautions (5.8)]
• Seizures [see Warnings and Precautions (5.9)]
• Elevated Cholesterol and Triglycerides [see Warnings and Precautions (5.10)]
• Hyponatremia [see Warnings and Precautions (5.11)]
• Transaminase Elevations [see Warnings and Precautions (5.12)]
• Discontinuation Syndrome [see Warnings and Precautions (5.13)]
• Use in Patients with Concomitant Illness [see Warnings and Precautions (5.14)]6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates
observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of
another drug and may not reflect the rates observed in practice.
The data described below are from clinical trials in which mirtazapine tablets were administered to
2796 patients in phase 2 and 3 clinical studies. The trials consisted of double-blind controlled and
open-label studies, inpatient and outpatient studies, fixed dose, and titration studies.
Adverse Reactions Leading to Discontinuation of Treatment
Approximately 16% of the 453 patients who received mirtazapine tablets in US 6-week
placebo-controlled clinical trials discontinued treatment due to an adverse reaction, compared to
7% of the 361 placebo-treated patients in those studies. The most common reactions leading to
discontinuation (>= and at a rate at least twice that of placebo) are included in Table 2.Table 2 : Adverse Reactions (≥1% and at least twice placebo) Leading to Discontinuation of Mirtazapine in 6-Week Clinical Trials in Patients with MDD
Mirtazapine Tablets
(n=453 )Placebo
(n=3 61)Somnolence 10.4% 2.2% Nausea 1.5% 0% Common Adverse Reactions
The most common adverse reactions (≥5% and twice placebo) associated with the use of
mirtazapine tablets are listed in Table 3.Table 3: Adverse Reactions (≥5% and twice placebo) in 6-Week U.S. Clinical Trials of
Mirtazapine in Patients with MDDMirtazapine Tablets
(n=453)Placebo
(n=3 61)Somnolence 54% 18% Increased Appetite 17% 2% Weight Gain 12% 2% Dizziness 7% 3% Table 4 enumerates adverse reactions that occurred in ≥1% of mirtazapine tablets-treated patients
and were more frequent than the placebo-treated patients, who participated in 6-week, U.S.
placebo-controlled trials in which patients were dosed in a range of 5 to 60 mg/day. This table
shows the percentage of patients in each group who had at least 1 episode of an adverse reaction
at some time during their treatment.Table 4:Adverse Reactions (≥1% and greater than placebo) in 6-Week U.S. Clinical Studies
of Mirtazapine in Patients with MDDMirtazapine Tablets
(n=453)Placebo
(n=361)Body as a Whole Asthenia 8% 5% Flu Syndrome 5% 3% Back Pain 2% 1% Digestive System Dry Mouth 25% 15% Increased Appetite 17% 2% Constipation 13% 7% Metabolic and Nutritional Disorders Weight Gain 12% 2% Peripheral Edema 2% 1% Edema 1% 0% Musculoskeletal System Myalgia 2% 1% Nervous System Somnolence 54% 18% Dizziness 7% 3% Abnormal Dreams 4% 1% Thinking Abnormal 3% 1% Tremor 2% 1% Confusion 2% 0% Respiratory System Dyspnea 1% 0% Urogenital System Urinary Frequency 2% 1% ECG Changes
The electrocardiograms for 338 patients who received mirtazapine tablets and 261 patients who
received placebo in 6-week, placebo-controlled trials were analyzed. Mirtazapine tablets was
associated with a mean increase in heart rate of 3.4 bpm, compared to 0.8 bpm for placebo. The
clinical significance of these changes is unknown.Other Adverse Events Observed During the Premarketing Evaluation of Mirtazapine Tablets
The following list does not include reactions: 1) already listed in previous tables or elsewhere in
labeling, 2) for which a drug cause was remote, 3) which were so general or excessively specific so
as to be uninformative, 4) which were not considered to have significant clinical implications, or 5)
which occurred at a rate equal to or less than placebo.
Adverse reactions are categorized by body system according to the following definitions: frequent
adverse reactions are those occurring in at least 1/100 patients; infrequent adverse reactions are
those occurring in 1/100 to 1/1000 patients; rare adverse reactions are those occurring in fewer
than 1/1000 patient.Body as a Whole
Frequent: malaise, abdominal pain, abdominal syndrome acute; infrequent: chills, fever, face edema, ulcer, photosensitivity reaction, neck rigidity, neck pain, abdomen enlarged; rare: cellulitis, chest pain substernal.
Cardiovascular System
Frequent: hypertension, vasodilatation; infrequent: angina pectoris, myocardial infarction, bradycardia, ventricular extrasystoles, syncope, migraine, hypotension; rare: atrial arrhythmia, bigeminy, vascular headache, pulmonary embolus, cerebral ischemia, cardiomegaly, phlebitis, left heart failure.
Digestive System
Frequent: vomiting, anorexia; infrequent: eructation, glossitis, cholecystitis, nausea and vomiting, gum hemorrhage, stomatitis, colitis, liver function tests abnormal; rare: tongue discoloration, ulcerative stomatitis, salivary gland enlargement, increased salivation, intestinal obstruction, pancreatitis, aphthous stomatitis, cirrhosis of liver, gastritis, gastroenteritis, oral moniliasis, tongue edema.
Hemic and Lymphatic System
Rare: lymphadenopathy, leukopenia, petechia, anemia, thrombocytopenia, lymphocytosis, pancytopenia.
Metabolic and Nutritional Disorders
Frequent: thirst; infrequent: dehydration, weight loss; rare: gout, SGOT increased, healing abnormal, acid phosphatase increased, SGPT increased, diabetes mellitus, hyponatremia.
Musculoskeletal System
Frequent: myasthenia, arthralgia; infrequent: arthritis, tenosynovitis; rare: pathologic fracture, osteoporosis fracture, bone pain, myositis, tendon rupture, arthrosis, bursitis.
Nervous System
Frequent: hypesthesia, apathy, depression, hypokinesia, vertigo, twitching, agitation, anxiety, amnesia, hyperkinesia, paresthesia; infrequent: ataxia, delirium, delusions, depersonalization, dyskinesia, extrapyramidal syndrome, libido increased, coordination abnormal, dysarthria, hallucinations, manic reaction, neurosis, dystonia, hostility, reflexes increased, emotional lability, euphoria, paranoid reaction; rare: aphasia, nystagmus, akathisia (psychomotor restlessness), stupor, dementia, diplopia, drug dependence, paralysis, grand mal convulsion, hypotonia, myoclonus, psychotic depression, withdrawal syndrome, serotonin syndrome.
Respiratory System
Frequent: cough increased, sinusitis; infrequent: epistaxis, bronchitis, asthma, pneumonia; rare: asphyxia, laryngitis, pneumothorax, hiccup.
Skin and Appendages
Frequent: pruritus, rash; infrequent: acne, exfoliative dermatitis, dry skin, herpes simplex, alopecia; rare: urticaria, herpes zoster, skin hypertrophy, seborrhea, skin ulcer.
Special Senses
Infrequent: eye pain, abnormality of accommodation, conjunctivitis, deafness, keratoconjunctivitis, lacrimation disorder, angle-closure glaucoma, hyperacusis, ear pain; rare: blepharitis, partial transitory deafness, otitis media, taste loss, parosmia.
Urogenital System
Frequent: urinary tract infection; infrequent: kidney calculus, cystitis, dysuria, urinary incontinence, urinary retention, vaginitis, hematuria, breast pain, amenorrhea, dysmenorrhea, leukorrhea, impotence; rare: polyuria, urethritis, metrorrhagia, menorrhagia, abnormal ejaculation, breast engorgement, breast enlargement, urinary urgency.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of mirtazapine
tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is
not always possible to reliably estimate their frequency or establish a causal relationship to drug
exposure.
Cardiac disorders: ventricular arrhythmia (Torsades de Pointes)
Endocrine disorders: hyperprolactinemia (and related symptoms, e.g., galactorrhea and
gynecomastia)
Musculoskeletal and connective tissue disorders: increased creatine kinase blood levels and
rhabdomyolysis
Psychiatric disorders: somnambulism (ambulation and other complex behaviors out of bed)
Reproductive system and breast disorder: priapism
Skin and subcutaneous tissue disorders: severe skin reactions, including DRESS, Stevens-Johnson
syndrome, bullous dermatitis, erythema multiforme and toxic epidermal necrolysis -
7 DRUG INTERACTIONS
Table 5 includes clinically important drug interactions with Mirtazapine Tablets [see Clinical
Pharmacology (12.3)].
Table 5: Clinically Important Drug Interactions with Mirtazapine Tablets Monoamine Oxidase Inhibitors (MAOIs) Clinical Impact The concomitant use of serotonergic drugs, including Mirtazapine Tablets, and
MAOIs increases the risk of serotonin syndrome.Intervention Mirtazapine tablets are contraindicated in patients taking MAOIs, including
MAOIs such as linezolid or intravenous methylene blue [see Dosage and
Administration (2.4), Contraindications (4), Warnings and Precautions (5.3)]Examples selegiline, tranylcypromine, isocarboxazid, phenelzine, linezolid, methylene blue Other Serotonergic Drugs Clinical Impact The concomitant use of serotonergic drugs with mirtazapine tablets increases
the risk of serotonin syndrome.Intervention Monitor patients for signs and symptoms of serotonin syndrome, particularly
during treatment initiation and dosage increases. If serotonin syndrome occurs,
consider discontinuation of mirtazapine tablets and/or concomitant serotonergic
drugs [see Warnings and Precautions (5.3)]Examples SSRIs, SNRIs, triptans, tricyclic antidepressants, fentanyl, lithium, amphetamines,
St. John’s Wort, tramadol, tryptophan, buspironeStrong CYP3A Inducers Clinical Impact The concomitant use of strong CYP3A inducers with mirtazapine tablets
decreases the plasma concentration of mirtazapine [see Clinical Pharmacology
(12.3)].Intervention Increase the dose of mirtazapine tablets if needed with concomitant CYP3A
inducer use. Conversely, a decrease in dosage of Mirtazapine Tablets may be
needed if the CYP3A inducer is discontinued [see Dosage and Administration
(2.5)].Examples phenytoin, carbamazepine, rifampin Strong CYP3A Inhibitors Clinical Impact The concomitant use of strong CYP3A inhibitors with mirtazapine tablets may
increase the plasma concentration of mirtazapine [see Clinical Pharmacology
(12.3)].Intervention Decrease the dose of mirtazapine tablets if needed with concomitant strong
CYP3A inhibitor use. Conversely, an increase in dosage of mirtazapine tablets
may be needed if the CYP3A inhibitor is discontinued [see Dosage and
Administration (2.5)].Examples itraconazole, ritonavir, nefazodone Cimetidine Clinical Impact The concomitant use of cimetidine, a CYP1A2, CYP2D6, and CYP3A inhibitor, with
mirtazapine tablets may increase the plasma concentration of mirtazapine [see
Clinical Pharmacology (12.3)]Intervention Decrease the dose of mirtazapine tablets if needed with concomitant cimetidine
use. Conversely, an increase in dosage of mirtazapine tablets may be needed if
cimetidine is discontinued [see Dosage and Administration (2.5)].Benzodiazepines and Alcohol Clinical Impact The concomitant use of benzodiazepines or alcohol with mirtazapine tablets
increases the impairment of cognitive and motor skills produced by mirtazapine
tablets alone.Intervention Avoid concomitant use of benzodiazepines and alcohol with mirtazapine tablets
[see Warnings and Precautions (5.7), Clinical Pharmacology (12.3)].Examples diazepam, alprazolam, alcohol Drugs that Prolong QTc Interval Clinical Impact The concomitant use of other drugs which prolong the QTc interval with
mirtazapine tablets, increase the risk of QT prolongation and/or ventricular
arrhythmias (e.g., Torsades de Pointes).Intervention Use caution when using mirtazapine tablets concomitantly with drugs that
prolong the QTc interval [see Warnings and Precautions (5.5), Clinical
Pharmacology (12.3)].Warfarin Clinical Impact The concomitant use of warfarin with mirtazapine tablets may result in an
increase in INR [see Clinical Pharmacology (12.3)].Intervention Monitor INR during concomitant use of warfarin with mirtazapine tablets -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to
antidepressants during pregnancy. Healthcare providers are encouraged to register patients by calling
the National Pregnancy Registry for Antidepressants at 1-844-405-6185 or visiting online at
https://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/antidepressants/.
Risk Summary
Prolonged experience with mirtazapine in pregnant women, based on published observational
studies and postmarketing reports, has not reliably identified a drug-associated risk of major birth
defects, miscarriage or adverse maternal or fetal outcomes. There are risks associated with
untreated depression in pregnancy (see Clinical Considerations).
In animal reproduction studies, oral administration of mirtazapine to pregnant rats and rabbits
during the period of organogenesis revealed no evidence of teratogenic effects up to 20 and 17
times the maximum recommended human dose (MRHD) of 45 mg, respectively, based on mg/m2
body surface area. However, in rats, there was an increase in post implantation loss at 20 times the
MRHD based on mg/m2 body surface area. Oral administration of mirtazapine to pregnant rats
during pregnancy and lactation resulted in an increase in pup deaths and a decrease in pup birth
weights at doses 20 times the MRHD based on mg/m2 body surface area (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population
is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse
outcomes. In the U.S. general population, the estimated background risk of major birth defects and
miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Women who discontinue antidepressants during pregnancy are more likely to experience a relapse
of major depression than women who continue antidepressants. This finding is from a prospective,
longitudinal study that followed 201 pregnant women with a history of major depressive disorder
who were euthymic and taking antidepressants at the beginning of pregnancy. Consider the risk of
untreated depression when discontinuing or changing treatment with antidepressant medication
during pregnancy and postpartum.Data
Animal Data
Mirtazapine was administered orally to pregnant rats and rabbits during the period of
organogenesis at doses of 2.5, 15, and 100 mg/kg/day and 2.5, 10, and 40 mg/kg/day,
respectively, which are up to 20 and 17 times the maximum recommended human dose (MRHD) of
45 mg based on mg/m2 body surface area, respectively. No evidence of teratogenic effects was
observed. However, in rats, there was an increase in post implantation loss in dams treated with
mirtazapine at 100 mg/kg/day which is 20 times the MRHD based on mg/m2 body surface area.
Oral administration of mirtazapine at doses of 2.5, 15, and 100mg/kg/day to pregnant rats during
pregnancy and lactation resulted in an increase in pup deaths during the first 3 days of lactation
and a decrease in pup birth weights at 20 times the MRHD based on mg/m2 body surface area. The
cause of these deaths is not known. The no effect dose level is 3 times the MRHD based on mg/m2
body surface area.8.2 Lactation
Risk Summary
Data from published literature report the presence of mirtazapine in human milk at low levels with
relative infant doses for mirtazapine ranging between 0.6 and 2.8% of the maternal
weight-adjusted dose (see Data). No adverse effects on the breastfed infant have been reported in
most cases of maternal use of mirtazapine. There are no data on the effects of mirtazapine on milk
production.
The developmental and health benefits of breastfeeding should be considered along with the
mother’s clinical need for mirtazapine and any potential adverse effects on the breastfed infant
from mirtazapine or from the underlying maternal condition.
Data
In a published pooled analysis of 8 breastfeeding mother-infant pairs, the mean (min, max) total
relative infant doses for mirtazapine and its desmethyl metabolite were 1.5% (0.6%, 2.8%) and
0.4% (0.1%, 0.7%) of the maternal weight-adjusted dose (median (min, max) dose of 38 mg
(30 mg, 120 mg), respectively). No adverse drug effects were reported for any of the infants.8.4 Pediatric Use
The safety and effectiveness of mirtazapine tablets have not been established in pediatric patients
with MDD. Two placebo-controlled trials in 258 pediatric patients with MDD have been conducted
with mirtazapine tablets, and the data were insufficient to establish the safety and effectiveness of
mirtazapine tablets in pediatric patients with MDD.
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric patients [see
Boxed Warning and Warnings and Precautions (5.1)].
In an 8-week-long clinical trial in pediatric patients receiving doses between 15 to 45 mg per day,
49% of mirtazapine tablets-treated patients had a weight gain of at least 7%, compared to 5.7% of
placebo- treated patients. The mean increase in weight was 4 kg (2 kg SD) for mirtazapine
tablets-treated patients versus 1 kg (2 kg SD) for placebo-treated patients [see Warnings and
Precautions (5.7)].8.5 Geriatric Use
Approximately 190 patients≥ 65 years of age participated in clinical studies with mirtazapine
tablets. Mirtazapine tablets are known to be substantially excreted by the kidney (75%), and the
risk of decreased clearance of this drug is greater in patients with impaired renal function.
Pharmacokinetic studies revealed a decreased clearance of mirtazapine in the elderly [see Clinical
Pharmacology (12.3)]
Sedating drugs, including mirtazapine tablets, may cause confusion and over-sedation in the
elderly. Elderly patients may be at greater risk of developing hyponatremia. Caution is indicated
when administering mirtazapine tablets to elderly patients [see Warnings and Precautions (5.12),
(5.15) and Clinical Pharmacology (12.3)]. In general, dose selection for an elderly patient should be
conservative, usually starting at the low end of the dosing range, reflecting the greater frequency of
decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.8.6 Renal or Hepatic Impairment
The clearance of mirtazapine is reduced in patients with moderate to severe renal or hepatic
impairment. Consequently, plasma mirtazapine levels may be increased in these patient groups,
compared to levels observed in patients without renal or hepatic impairment. Dosage decrease may
be necessary when administering mirtazapine tablets to patients with moderate to severe renal or
hepatic impairment [seeWarnings and Precautions(5.13),Use in Specific Populations(8.5), and
Clinical Pharmacology(12.3)]. -
10 OVERDOSAGE
Human Experience
In premarketing clinical studies, there were reports of mirtazapine tablets overdose alone or in
combination with other pharmacological agents. Signs and symptoms reported in association with
overdose included disorientation, drowsiness, impaired memory, and tachycardia.
Based on postmarketing reports, serious outcomes (including fatalities) may occur at dosages
higher than the recommended doses, especially with mixed overdoses. In these cases, QT
prolongation and Torsades de Pointes have also been reported [see Warnings and Precautions (5.5),
Adverse Reactions (6.2), and Drug Interactions (7)].Overdose Management
No specific antidotes for mirtazapine are known.
Contact Poison Control (1-800-222-1222) for the latest recommendations. -
11 DESCRIPTION
Mirtazapine tablets contain mirtazapine. Mirtazapine has a tetracyclic chemical structure and
belongs to the piperazino-azepine group of compounds. It is designated
1,2,3,4,10,14b-hexahydro-2-methylpyrazino [2,1-a] pyrido [2,3-c][2] benzazepine and has the
empirical formula of C17H19N3. Its molecular weight is 265.35. The structural formula is the
following and it is the racemic mixture: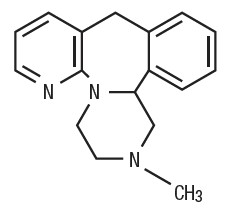
Mirtazapine is a white to creamy white crystalline powder which is practically insoluble in water.
Mirtazapine tablets are available for oral administration as scored film-coated tablets containing 15
or 30 mg of mirtazapine. Each tablet contains the following inactive ingredients: croscarmellose
sodium, hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate,
microcrystalline cellulose, polyethylene glycol and titanium dioxide. In addition, mirtazapine tablets,
USP 15 mg and 30 mg contains iron oxide yellow and mirtazapine tablets, USP 30 mg contains iron
oxide red -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of mirtazapine for the treatment of major depressive disorder, is unclear.
However, its efficacy could be mediated through its activity as an antagonist at central presynaptic
α2-adrenergic inhibitory auto-receptors and heteroreceptors and enhancing central noradrenergic
and serotonergic activity.12.2 Pharmacodynamics
In preclinical studies, mirtazapine acts as an antagonist at α2-adrenergic inhibitory auto-receptors
and heteroreceptors and as an antagonist at serotonin 5-HT2 and 5-HT3 receptors. Mirtazapine has
no significant affinity for the 5-HT1A and 5-HT1B receptors.
Mirtazapine also acts as an antagonist of histamine (H1) receptors, peripheral α1-adrenergic
receptors, and muscarinic receptors. Actions at these receptors may explain some of the other
clinical effects of mirtazapine (e.g., its prominent somnolent effects and orthostatic hypotension
may be explained by its inhibition of histamine (H1) receptors and peripheral α1-adrenergic
receptors, respectively).
Cardiac Electrophysiology
The effect of mirtazapine tablets on QTc interval was assessed in healthy subjects. At a dose of
75 mg (1.67 times the maximum recommended dosage), mirtazapine tablets do not prolong the
QTc interval to a clinically meaningful extent.12.3 Pharmacokinetics
Plasma levels of mirtazapine are linearly related to dose over a dose range of 15 to 80 mg (1.78
times the maximum recommended dose). Steady state plasma levels of mirtazapine are attained
within 5 days, with about 50% accumulation (accumulation ratio=1.5). The (–) enantiomer has an
elimination half-life that is approximately twice as long as the (+) enantiomer and therefore
achieves plasma levels that are about 3 times as high as that of the (+) enantiomer.
Absorption
Mirtazapine has an absolute bioavailability of about 50% following oral administration. Peak plasma
concentrations of mirtazapine are reached within about 2 hours post dose.
Food Effect
The presence of food in the stomach has a minimal effect on both the rate and extent of absorption.
Distribution
Mirtazapine is approximately 85% bound to plasma proteins over a concentration range of 0.01 to
10 mcg/mL.
Elimination
Mirtazapine has a half-life of about 20 to 40 hours following oral administration of mirtazapine
tabletsMetabolism
Mirtazapine is extensively metabolized after oral administration. Major pathways of
bio-transformation are demethylation and hydroxylation followed by glucuronide conjugation. In
vitro data from human liver microsomes indicate that CYP2D6 and CYP1A2 are involved in the
formation of the 8-hydroxy metabolite of mirtazapine, whereas CYP3A is considered to be
responsible for the formation of the N-desmethyl and N-oxide metabolite. Several unconjugated
metabolites possess pharmacological activity but are present in the plasma at very low levels.
Excretion
Mirtazapine and its metabolites are eliminated predominantly (75%) via urine with 15% in feces.
Specific Populations
Geriatric Patients
Following oral administration of mirtazapine tablets 20 mg/day for 7 days to subjects of varying
ages (range 25 to 74 years old), oral clearance of mirtazapine was reduced in the elderly compared
to the younger subjects. The clearance in elderly males was 40% lower compared to younger
males, while the clearance was 10% lower in elderly females compared to younger females [see
Warnings and Precautions (5.14), Use in Specific Populations (8.5)]Male and Female Patients
The mean elimination half-life of mirtazapine after oral administration ranges from approximately
20 to 40 hours across age and gender subgroups, with females of all ages exhibiting significantly
longer elimination half-lives than males (mean half-life of 37 hours for females vs. 26 hours for
males).
Race
There have been no clinical studies to evaluate the effect of race on the pharmacokinetics of
mirtazapine tablets.
Patients with Renal Impairment
When compared to subjects with normal renal function, total body clearance of mirtazapine was
reduced approximately 30% in renal impaired patients with GFR=11–39 mL/min/1.73 m2 and
approximately 50% in renal impaired patients with GFR=<10 mL/min/1.73 m2) [see Warnings and
Precautions (5.14), Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
Following a single 15-mg oral dose of mirtazapine tablets, the oral clearance of mirtazapine in
patients with hepatic impairment was decreased by approximately 30%, compared to subjects with
normal hepatic function [see Warnings and Precautions (5.12, 5.14), Use in Specific Populations
(8.6)].Drug Interactions Studies
Warfarin
Mirtazapine (30 mg daily) at steady state caused a statistically significant increase (0.2) in the
International Normalized Ratio (INR) in subjects treated with warfarin [see Drug Interactions (7)].
QTc-Prolonging Drugs
The risk of QT prolongation and/or ventricular arrhythmias (e.g., Torsades de Pointes) may be
increased with concomitant use of medicines which prolong the QTc interval (e.g., some
antipsychotics and antibiotics) and in mirtazapine overdose [see Warnings and Precautions (5.5),
Adverse Reactions (6.1,6.2), Drug Interactions (7), and Overdosage (10)].
Phenytoin
In healthy male subjects (n=18), phenytoin (200 mg daily, at steady state) increased mirtazapine
(30 mg daily, at steady state) clearance about 2-fold, resulting in a decrease in average plasma
mirtazapine concentrations of 45% [see Drug Interactions (7)]. Mirtazapine did not significantly
affect the pharmacokinetics of phenytoin.
Carbamazepine
In healthy male subjects (n=24), carbamazepine (400 mg twice a day, at steady state) increased
mirtazapine (15 mg twice a day, at steady state) clearance about 2-fold, resulting in a decrease in
average plasma mirtazapine concentrations of 60% [see Drug Interactions (7)].Cimetidine
In healthy male subjects (n=12), when cimetidine, a weak inhibitor of CYP1A2, CYP2D6, and
CYP3A4, given at 800 mg b.i.d. at steady state was coadministered with mirtazapine (30 mg daily)
at steady state, the Area Under the Curve (AUC) of mirtazapine increased more than 50% [see Drug
Interactions (7)]. Mirtazapine did not cause relevant changes in the pharmacokinetics of cimetidine.
Ketoconazole
In healthy male Caucasian subjects (n=24), coadministration of the strong CYP3A4 inhibitor
ketoconazole (200 mg b.i.d. for 6.5 days) increased the peak plasma levels and the AUC of a single
30 mg dose of mirtazapine by approximately 40% and 50%, respectively [see Drug Interactions (7)].
Amitriptyline
In healthy, CYP2D6 extensive metabolizer patients (n=32), amitriptyline (75 mg daily), at steady
state, did not cause relevant changes to the pharmacokinetics of steady state mirtazapine (30 mg
daily); mirtazapine also did not cause relevant changes to the pharmacokinetics of amitriptyline.
Paroxetine
In healthy CYP2D6 extensive metabolizer subjects (n=24), mirtazapine (30 mg/day), at steady state,
did not cause relevant changes in the pharmacokinetics of steady state paroxetine (40 mg/day), a
CYP2D6 inhibitor.Lithium
No relevant clinical effects or significant changes in pharmacokinetics have been observed in
healthy male subjects on concurrent treatment with lithium 600 mg/day for 10 days at steady state
and a single 30 mg dose of mirtazapine. The effects of higher doses of lithium on the
pharmacokinetics of mirtazapine are unknown.
Risperidone
Mirtazapine (30 mg daily) at steady state did not influence the pharmacokinetics of risperidone (up
to 3 mg twice a day) in subjects (n=6) in need of treatment with an antipsychotic and
antidepressant drug.
Alcohol
Concomitant administration of alcohol (equivalent to 60 g) had a minimal effect on plasma levels of
mirtazapine (15 mg) in 6 healthy male subjects. However, the impairment of cognitive and motor
skills produced by mirtazapine tablets were shown to be additive with those produced by alcohol.
Diazepam
Concomitant administration of diazepam (15 mg) had a minimal effect on plasma levels of
mirtazapine (15 mg) in 12 healthy subjects. However, the impairment of motor skills produced by
mirtazapine tablets has been shown to be additive with those caused by diazepam. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies were conducted with mirtazapine given in the diet at doses of 2, 20, and
200 mg/kg/day to mice and 2, 20, and 60 mg/kg/day to rats. The highest doses used are
approximately 20 and 12 times the maximum recommended human dose (MRHD) of 45 mg/day,
based on body surface area (mg/m2) in mice and rats, respectively. There was an increased
incidence of hepatocellular adenoma and carcinoma in male mice at the high dose. In rats, there
was an increase in hepatocellular adenoma in females at the mid and high doses and in
hepatocellular tumors and thyroid follicular adenoma/cystadenoma and carcinoma in males at the
high dose.
Mutagenesis
Mirtazapine was not mutagenic or clastogenic and did not induce general DNA damage as
determined in several genotoxicity tests: Ames test, in vitro gene mutation assay in Chinese
hamster V 79 cells, in vitro sister chromatid exchange assay in cultured rabbit lymphocytes, in vivo
bone marrow micronucleus test in rats, and unscheduled DNA synthesis assay in HeLa cells.
Impairment of Fertility
In a fertility study in rats, mirtazapine was given at doses up to 100 mg/kg [20 times the maximum
recommended human dose (MRHD), based on body surface area (mg/m2)]. Mating and conception
were not affected by the drug, but estrous cycling was disrupted at doses that were 3 or more
times the MRHD, and pre-implantation losses occurred at 20 times the MRHD. -
14 CLINICAL STUDIES
The efficacy of mirtazapine tablets as a treatment for major depressive disorder was established in
4 placebo- controlled, 6-week trials in adult outpatients meeting DSM-III criteria for major
depressive disorder. Patients were titrated with mirtazapine tablets from a dose range of 5 mg to
35 mg/day. The mean mirtazapine dose for patients who completed these 4 studies ranged from 21
to 32 mg/day. Overall, these studies demonstrated mirtazapine tablets to be superior to placebo on
at least 3 of the following 4 measures: 21-Item Hamilton Depression Rating Scale (HDRS) total
score; HDRS Depressed Mood Item; CGI Severity score; and Montgomery and Asberg Depression
Rating Scale (MADRS). Superiority of mirtazapine tablets over placebo was also found for certain
factors of the HDRS, including anxiety/somatization factor and sleep disturbance factor.
Examination of age and gender subsets of the population did not reveal any differential
responsiveness on the basis of these subgroupings.
In a longer-term study, patients meeting (DSM-IV) criteria for major depressive disorder who had
responded during an initial 8 to 12 weeks of acute treatment on mirtazapine tablets were
randomized to continuation of mirtazapine tablets or placebo for up to 40 weeks of observation for
relapse. Response during the open phase was defined as having achieved a HAM-D 17 total score
of
≤8 and a CGI-Improvement score of 1 or 2 at 2 consecutive visits beginning with week 6 of the 8
to 12 weeks in the open-label phase of the study. Relapse during the double-blind phase was
determined by the individual investigators. Patients receiving continued mirtazapine tablets
treatment experienced significantly lower relapse rates over the subsequent 40 weeks compared to
those receiving placebo. This pattern was demonstrated in both male and female patients. -
16 HOW SUPPLIED/STORAGE AND HANDLING
Mirtazapine tablets are supplied as:
Mirtazapine Tablets, USP 15 mg are available for oral administration as pale yellow, oval-shaped,
scored, film-coated tablets imprinted “APO” on one side and “MI” bisect “15” on the other side.
They are supplied as follows:
Boxes of 10x10 UD 100 count NDC 63739-098-10
Mirtazapine Tablets, USP 30 mg are available for oral administration as light pink, oval-shaped,
scored, film-coated tablets imprinted “APO” on one side and “MI” bisect “30” on the other side.
They are supplied as follows:
Boxes of 10x10 UD 100 count NDC 63739-099-10
Storage
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see
USP Controlled Room Temperature]. Protect from light and moisture. -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Suicidal Thoughts and Behaviors
Advise patients and caregivers to look for the emergence of suicidality, especially early during
treatment and when the dosage is adjusted up or down, and instruct them to report such symptoms
to the healthcare provider [see Boxed Warning and Warnings and Precautions (5.1)].
Agranulocytosis
Advise patients to contact their physician if they experience fever, chills, sore throat, mucous
membrane ulceration, flu-like complaints, or other symptoms that might suggest infection [see
Warnings and Precautions (5.2)].
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome, particularly with the concomitant use of
mirtazapine tablets with other serotonergic drugs including triptans, tricyclic antidepressants,
fentanyl, lithium, tramadol, tryptophan, buspirone, amphetamines, St. John’s Wort, and with drugs
that impair metabolism of serotonin (in particular, MAOIs, both those intended to treat psychiatric
disorders and also others, such as linezolid). Advise patients to contact their healthcare provider or
report to the emergency room if they experience signs or symptoms of serotonin syndrome [see
Dosage and Administration (2.4), Contraindications (4), Warnings and Precautions (5.3), Drug
Interactions (7)].QT Prolongation and Torsades de Pointes
Inform patients to consult their physician immediately if they feel faint, lose consciousness, or have
heart palpitations [see Warnings and Precautions (5.5), Drug Interactions (7), Overdosage (10)].
Advise patients to inform physicians that they are taking mirtazapine tablets before any new drug is
taken.Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
Advise patients to report to their healthcare provider at the earliest onset of fever, rash, swollen
lymph nodes, or other signs and symptoms suggestive of Drug Reaction with Eosinophilia and
Systemic Symptoms (DRESS) [see Contraindications (4), Warnings and Precautions (5.6)].
Somnolence
Advise patients that mirtazapine tablets may impair judgment, thinking, and particularly, motor
skills, because of its prominent sedative effect. Caution patients about performing activities
requiring mental alertness, such as operating hazardous machinery or operating a motor vehicle,
until they are reasonably certain that mirtazapine tablets therapy does not adversely affect their
ability to engage in such activities. [see Warnings and Precautions (5.7)].
Alcohol
Advise patients to avoid alcohol while taking mirtazapine tablets [see Warnings and Precautions
(5.7), Drug Interactions (7)].
Activation of Mania/Hypomania
Advise patients and their caregivers to observe for signs of activation of mania/hypomania and
instruct them to report such symptoms to the healthcare provider [see Warnings and Precautions
(5.8)].Discontinuation Syndrome
Advise patients not to abruptly discontinue mirtazapine tablets and to discuss any tapering regimen
with their healthcare provider. Adverse reactions can occur when mirtazapine tablets are
discontinued [see Dosage and Administration (2.6), Warnings and Precautions (5.13)].
Allergic Reactions
Advise patients to notify their healthcare provider if they develop an allergic reaction such as rash,
hives, swelling, or difficulty breathing [see Contraindications (4), Adverse Reactions (6.2)].
Pregnancy
• Advise patients to notify their physician if they become pregnant or intend to become pregnant
during mirtazapine tablets therapy.
• Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes
in women exposed to mirtazapine tablets during pregnancy [see Use in Specific Populations
(8.2)].
Lactation
Advise patients to notify their physician if they are breastfeeding an infant [see Use in Specific
Populations (8.2)].Angle-Closure Glaucoma
Patients should be advised that taking mirtazapine can cause mild pupillary dilation, which in
susceptible individuals, can lead to an episode of angle-closure glaucoma. Pre-existing glaucoma is
almost always open-angle glaucoma because angle-closure glaucoma, when diagnosed, can be
treated definitively with iridectomy. Open-angle glaucoma is not a risk factor for angle-closure
glaucoma. Patients may wish to be examined to determine whether they are susceptible to
angle-closure, and have a prophylactic procedure (e.g., iridectomy), if they are susceptible [see
Warnings and Precautions (5.4).]Dispense with Medication Guide available at www1.apotex.com/products/us
Distributed By:
McKesson Corporation dba SKY Packaging
Memphis, TN 38141
Manufactured by:
Apotex Inc
Toronto, Ontario
Canada M9L 1T9
Revised: 02/2024
21495-2 -
MEDICATION GUIDE
Mirtazapine Tablets, USP
(mir taz’ a peen)Medication Guide available at www1.apotex.com/products/us
What is the most important information I should know
about mirtazapine tablets?
Mirtazapine tablets may cause serious side effects, including:
• Increased risk of suicidal thoughts or actions in
some children and young adults. Mirtazapine tablets,
and other antidepressant medicines may increase
suicidal thoughts or actions in some people 24 years of
age and younger, especially within the first few
months of treatment or when the dose is changed.
Mirtazapine tablets are not for use in children.
° Depression or other serious mental illnesses are
the most important causes of suicidal thoughts orHow can I watch for and try to prevent suicidal
thoughts and actions?° Pay close attention to any changes, especially sudden
changes in mood, behavior, thoughts, or feelings, or if
you develop suicidal thoughts or actions. This is very
important when an antidepressant medicine is started
or when the dose is changed.
° Call your healthcare provider right away to report new
or sudden changes in mood, behavior, thoughts, or
feelings.
° Keep all follow-up visits with your healthcare provider
as scheduled. Call your healthcare provider between
visits as needed, especially if you have concerns
about symptoms.Call your healthcare provider or get emergency medical
help right away if you or your family member have any
of the following symptoms, especially if they are new,
worse, or worry you:• attempts to commit suicide
• acting aggressive, being angry or violent
• new or worse depression
• panic attacks
• new or worse irritability
• an extreme increase in activity or talking (mania)
• acting on dangerous impulses
• thoughts about suicide or dying
• new or worse anxiety
• feeling very agitated or restless
• trouble sleeping
• other unusual changes in behavior or moodWhat are mirtazapine tablets?
Mirtazapine tablets are prescription medicines used to treat
a certain type of depression called Major Depressive
Disorder (MDD) in adults.
It is not known if mirtazapine tablets are safe and effective
for use to treat MDD in children.Who should not take mirtazapine tablets? Do not take
mirtazapine tablets if you:
• take a Monoamine Oxidase Inhibitor (MAOI)
• have stopped taking an MAOI in the last 14 days
• are being treated with the antibiotic linezolid or
intravenous methylene blue
• if you are allergic to mirtazapine or any of the ingredients
in mirtazapine tablets. See the end of this Medication
Guide for a complete list of ingredients in mirtazapine
tabletsAsk your healthcare provider or pharmacist if you are not
sure if you take an MAOI, including the antibiotic linezolid or
intravenous methylene blue.Do not start taking an MAOI for at least 14 days after
you stop treatment with mirtazapine tablets.
Before taking mirtazapine tablets, tell your healthcare
provider about all your medical conditions, including if you:• have a history of suicide or depression
• have a history or family history of bipolar disorder, mania
or hypomania
• have a low white blood cell count
• have glaucoma (high pressure in the eye)
• have or had heart problems or stroke
• have an abnormal heart beat called QT prolongation or a
family history of QT prolongation
• have seizures
• have high cholesterol or triglyceride levels
• have low sodium levels in your blood
• have or had kidney or liver problems
• have low blood pressure
• are pregnant or plan to become pregnant. It is not known
if mirtazapine tablets will harm your unborn baby.• Talk to your healthcare provider if you become pregnant
or think you may be pregnant during treatment with
mirtazapine tablets.
• If you become pregnant while taking mirtazapine tablets,
talk to your healthcare provider about registering with
the National Pregnancy Registry for Antidepressants. You
can register by calling 1-844-405-6185 or visiting online
at http://womensmentalhealth.org/clinical-and-researchprograms/
pregnancyregistry/antidepressants/. The
purpose of this registry is to monitor the pregnancy
outcomes in women who have been treated with
mirtazapine tablets at any time during pregnancy.
• are breastfeeding or plan to breastfeed. Mirtazapine may
pass into your breast milk. Talk to your healthcare
provider about the best way to feed your baby during
treatment with mirtazapine tablets.Tell your healthcare provider about all the medicines
you take, including prescription and over-the-counter
medicines, vitamins, and herbal supplements.
Mirtazapine tablets and other medicines may affect each
other causing possible serious side effects.
Mirtazapine tablets may affect the way other medicines
work and other medicines may affect the way mirtazapine
tablets work.Especially tell your healthcare provider if you take:
• MAOIs
• medicines to treat migraine headaches known as
triptans
• tricyclic antidepressants
• fentanyl
• lithium
• tramadol
• tryptophan
• buspirone
• amphetamines
• benzodiazepines
• St. John’s Wort• medicines used to treat mood, anxiety, psychotic or
thought disorders, including selective serotonin reuptake
inhibitors (SSRIs) and serotonin norepinephrine reuptake
inhibitors (SNRIs)
• medicines that may affect your heart rhythm (such as
certain antibiotics and some antipsychotics)
Ask your healthcare provider if you are not sure if you are
taking any of these medicines. Your healthcare provider can
tell you if it is safe to take mirtazapine tablets with your
other medicines.Do not start or stop any other medicines during treatment
with mirtazapine tablets without talking to your healthcare
provider first. Stopping mirtazapine tablets suddenly may
cause you to have serious side effects. See, "What are the
possible side effects of mirtazapine tablets?"
Know the medicines you take. Keep a list of them to show
to your healthcare provider and pharmacist when you get a
new medicine.How should I take mirtazapine tablets?
• Take mirtazapine tablets exactly as your healthcare
provider tells you to. Do not change your dose or stop
taking mirtazapine tablets without first talking to your
healthcare provider.
• Your healthcare provider may need to change the dose of
mirtazapine tablets until it is the right dose for you
• Take mirtazapine tablets 1 time each day, preferably in
the evening at bedtime
• If you take too much mirtazapine tablets call your
healthcare provider or poison control center at
1-800-222-1222 right away or go to the nearest hospital
emergency room.What should I avoid while taking mirtazapine tablets?
• Do not drive, operate heavy machinery, or do other
dangerous activities until you know how mirtazapine
tablets affects you. Mirtazapine tablets can cause
sleepiness or may affect your ability to make decisions,
think clearly, or react quickly.
• Avoid drinking alcohol during treatment with mirtazapine
tablets.
• Avoid taking medicines used to treat anxiety, insomnia,
and seizures, called benzodiazepines, during treatment
with mirtazapine tablets. Ask your healthcare provider if
you are not sure if you take one of these medicines.What are the possible side effects of mirtazapine tablets?
Mirtazapine tablets may cause serious side effects,
including:
• See, "What is the most important information I
should know about mirtazapine?"
• Low white blood cell count. Tell your healthcare
provider right away if you develop any signs or
symptoms of a low white blood cell count, including:
° fever
° sore throat
° flu-like symptoms
° chills
° mouth and nose sores
° infections• Serotonin syndrome. A potentially
life-threatening problem called serotonin syndrome can
happen when you take mirtazapine tablets with certain
other medicines. See, "Who should not take
mirtazapine tablets?" Stop taking mirtazapine tablets
and call your healthcare provider or go to the nearest
hospital emergency room right away if you have any of
the following signs and symptoms of serotonin
syndrome:° agitation
° confusion
° fast heart beat
° dizziness
° flushing
° tremors, stiff muscles, or muscle twitching
° seizures
° seeing or hearing things that are not real
(hallucinations)
° coma
° blood pressure changes
° sweating
° high body temperature (hyperthermia)
° loss of coordination nausea,
° vomiting, diarrhea• Eye problems (angle-closure glaucoma). Mirtazapine
tablets may cause a certain type of eye problem called
angle-closure glaucoma. Call your healthcare provider if
you have eye pain, changes in your vision, or swelling or
redness in or around the eye. Only some people are at
risk for these problems. You may want to undergo an eye
examination to see if you are at risk and receive
preventative treatment if you are.• Heart rhythm problems.
• Severe skin reaction. Mirtazapine tablets may cause a
severe skin reaction that may include rash, fever, swollen
glands, and other organ involvement such as liver,
kidney, lung and heart. The reaction may sometimes be
fatal. Tell your healthcare provider right away if you
experience any of these signs.
• Increased appetite and weight gain.
• Sleepiness. See, "What should I avoid while taking
mirtazapine tablets?"
• Mania or hypomania (manic episodes) in people who
have a history of bipolar disorder. Symptoms may
include:° greatly increased energy
° racing thoughts
° unusually grand ideas
° talking more or faster than usual
° severe trouble sleeping
° reckless behavior
° excessive happiness or irritability• Seizures (convulsions).
• Increased fat levels (cholesterol and triglycerides) in
your blood.
• Low sodium levels in your blood (hyponatremia). Low
sodium levels in your blood may be serious and may
cause death. Elderly people may be at greater risk for
this. Signs and Symptoms of low sodium levels in your
blood may include:
° headache
° memory changes
° weakness and unsteadiness on your feet which can
lead to falls
° difficulty concentrating
° confusionIn severe or more sudden cases, signs and symptoms
include:
° hallucinations (seeing or hearing things that are not
real)
° seizures
° respiratory arrest
° fainting
° coma
° death• Changes in liver function tests.
• Discontinuation syndrome. Suddenly stopping
mirtazapine tablets may cause you to have serious side
effects. Your healthcare provider may want to decrease
your dose slowly. Symptoms may include:
° dizziness
° irritability and agitation
° anxiety
° sweating
° seizures
° ringing in your ears (tinnitus)
° nausea and vomiting
° problems sleeping
° tiredness
° confusion° electric shock sensation (paresthesia)
° shaking (tremor)
° headache
° abnormal dreams
° changes in your mood
° hypomaniaThe most common side effects of mirtazapine tablets
include:
• sleepiness
• increased appetite
• weight gain
• dizziness
These are not all the possible side effects of mirtazapine
tablets.
Call your doctor for medical advice about side effects. You
may report side effects to FDA at 1-800-FDA-1088.
How should I store mirtazapine tablets?
• Store mirtazapine tablets at room temperature between
68°F to 77°F (20°C to 25°C).
• Keep mirtazapine tablets away from light and moisture.Keep mirtazapine tablets, and all medicines out of the
reach of children.
General information about the safe and effective use of
mirtazapine tablets.Medicines are sometimes prescribed for purposes other
than those listed in a Medication Guide. Do not use
mirtazapine tablets for a condition for which it was not
prescribed. Do not give mirtazapine tablets to other people,
even if they have the same symptoms that you have. It may
harm them. You can ask your healthcare provider or
pharmacist for information about mirtazapine tablets that is
written for healthcare professionals.What are the ingredients in mirtazapine tablets?
Active ingredient: mirtazapine
Inactive ingredients:15 mg tablets: Croscarmellose sodium, hydroxypropyl
cellulose, hypromellose, lactose monohydrate, magnesium
stearate, microcrystalline cellulose, polyethylene glycol,
titanium dioxide and iron oxide yellow.
30 mg tablets: Croscarmellose sodium, hydroxypropyl
cellulose, hypromellose, lactose monohydrate, magnesium
stearate, microcrystalline cellulose, polyethylene glycol,
titanium dioxide and iron oxide yellow and iron oxide red.Distributed By:
McKesson Corporation dba SKY Packaging
Memphis, TN 38141
Manufactured By:
Apotex Inc.
Toronto, Ontario
Canada M9L 1T9
Revised: 02/2024
21495-2 - MIRTAZAPINE
- MIRTAZAPINE
-
INGREDIENTS AND APPEARANCE
MIRTAZAPINE
mirtazapine tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63739-098(NDC:60505-0247) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MIRTAZAPINE (UNII: A051Q2099Q) (MIRTAZAPINE - UNII:A051Q2099Q) MIRTAZAPINE 15 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color yellow Score 2 pieces Shape OVAL Size 9mm Flavor Imprint Code APO;MI;15 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63739-098-10 10 in 1 BOX 11/15/2019 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077666 08/22/2007 MIRTAZAPINE
mirtazapine tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63739-099(NDC:60505-0248) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MIRTAZAPINE (UNII: A051Q2099Q) (MIRTAZAPINE - UNII:A051Q2099Q) MIRTAZAPINE 30 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color pink Score 2 pieces Shape OVAL Size 11mm Flavor Imprint Code APO;MI;30 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63739-099-10 10 in 1 BOX 11/15/2019 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077666 08/22/2007 Labeler - McKesson Corporation dba SKY Packaging (140529962) Establishment Name Address ID/FEI Business Operations Legacy Pharmaceutical Packaging, LLC 143213275 repack(63739-098, 63739-099) , relabel(63739-098, 63739-099)




