Label: NALTREXONE implant
-
Contains inactivated NDC Code(s)
NDC Code(s): 69364-3143-6 - Packager: Complete Pharmacy and Medical Solutions
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 19, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
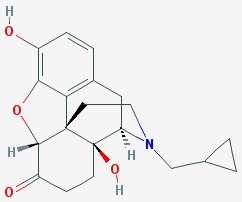
DescriptionNaltrexone Implant - Formula:17-(Cyclopropilmethil)-4,5ª-Epoxy-3,14-dihidroximorfinan-6-ona - Formula molecular C20H23NO4C20H23NO4 - Naltrexone Implants are supplied in a kit containing 6 X ...
-
Clinical PharmacologyMechanism of Action - Naltrexone is an opioid antagonist with highest affinity for the mµ opioid receptor. Naltrexone has few, if any, intrinsic actions besides its opioid blocking properties ...
-
PharmacokineticsAbsorption - Naltrexone Implants are intended to release over a 10-12 week period when administered subcutaneously. After implantation, the naltrexone plasma concentration time profile is ...
-
Clinical StudiesMultiple studies have demonstrated an increase of adherence to drug and alcohol detox programs with extended release formulations. The efficacy of naltrexone implants in the treatment of alcohol ...
-
Indications and UsageTreatment with naltrexone implants should be part of a comprehensive management program that includes psychological and psychosocial support. Naltrexone tablets were initially approved by the FDA ...
-
Dosage and AdministrationNaltrexone implants must be administered by a physician. To prevent occurrence of an acute abstinence syndrome (withdrawal) in patients dependent on opioids, or exacerbation of a pre-existing ...
-
Laboratory TestsHepatic Enzymes - In short-term, controlled trials, in alcohol-dependent patients, the incidence of AST elevations associated with Naltrexone Injection treatment was similar to that observed ...
-
Platelet Count.Extended release Naltrexone formulations have reported a decrease in platelet count, although these reports were not associated with an increase in bleeding-related adverse events. Creatinine ...
-
Warnings and PrecautionsHepatoxicity - Naltrexone has the capacity to cause hepatocellular injury when given in excessive doses. Naltrexone is contraindicated in acute hepatitis or liver failure, and its use ...
-
ContraindicationsNaltrexone is contraindicated in: Patients receiving opioid analgesics (see PRECAUTIONS). Patients with current physiologic opioid dependence (see WARNINGS). Patients in acute opiate withdrawal ...
-
Adverse EffectsNaltrexone may cause side effects including: A reaction at the implant site. The reaction could be pain, tenderness, swelling, redness, and/or itching. Nausea - The other common side effects of ...
-
Drug InteractionsPatients taking naltrexone may not benefit from opioid-containing medicines (see PRECAUTIONS, Pain Management). Because naltrexone is not a substrate for CYP drug metabolizing enzymes, inducers or ...
-
Carcinogenesis, Mutagenesis, Impairment of FertilityCarcinogenicity studies have not been conducted with naltrexone implants. Carcinogenicity studies of oral naltrexone hydrochloride (administered via the diet) have been conducted in rats and mice ...
-
Pregnancy Category CReproduction and developmental studies have not been conducted for naltrexone implants. Studies with naltrexone administered via the oral route have been conducted in pregnant rats and ...
-
Labor and DeliveryThe potential effect of naltrexone implants on duration of labor and delivery in humans is unknown.
-
Nursing MothersTransfer of naltrexone and 6β-naltrexol into human milk has been reported with oral naltrexone. Because of the potential for tumorigenicity shown for naltrexone in animal studies, and because of ...
-
Pediatric UseThe safety and efficacy of naltrexone implants have not been established in the pediatric population.
-
Geriatric useClinical studies of naltrexone implants did not include sufficient numbers of subjects age 65 and over to determine whether they respond differently from younger subjects.
-
How SuppliedNaltrexone implants are available in a box of 6 X 200mg Naltrexone implants. Each implant is individually blister packed. Storage - Store at 20° to 25°C (68° to 77°F); excursions permitted to 15 ...
-
Naltrexone Implant Patient InformationWhat is Naltrexone Implant? Naltrexone blocks the effects of narcotic medicines and alcohol. Naltrexone implants are used to treat addiction to alcohol or narcotic drugs. It is also used to ...
-
PRINCIPAL DISPLAY PANEL - NDC: 69364-3143-6 - 6-count Box Label

-
INGREDIENTS AND APPEARANCEProduct Information