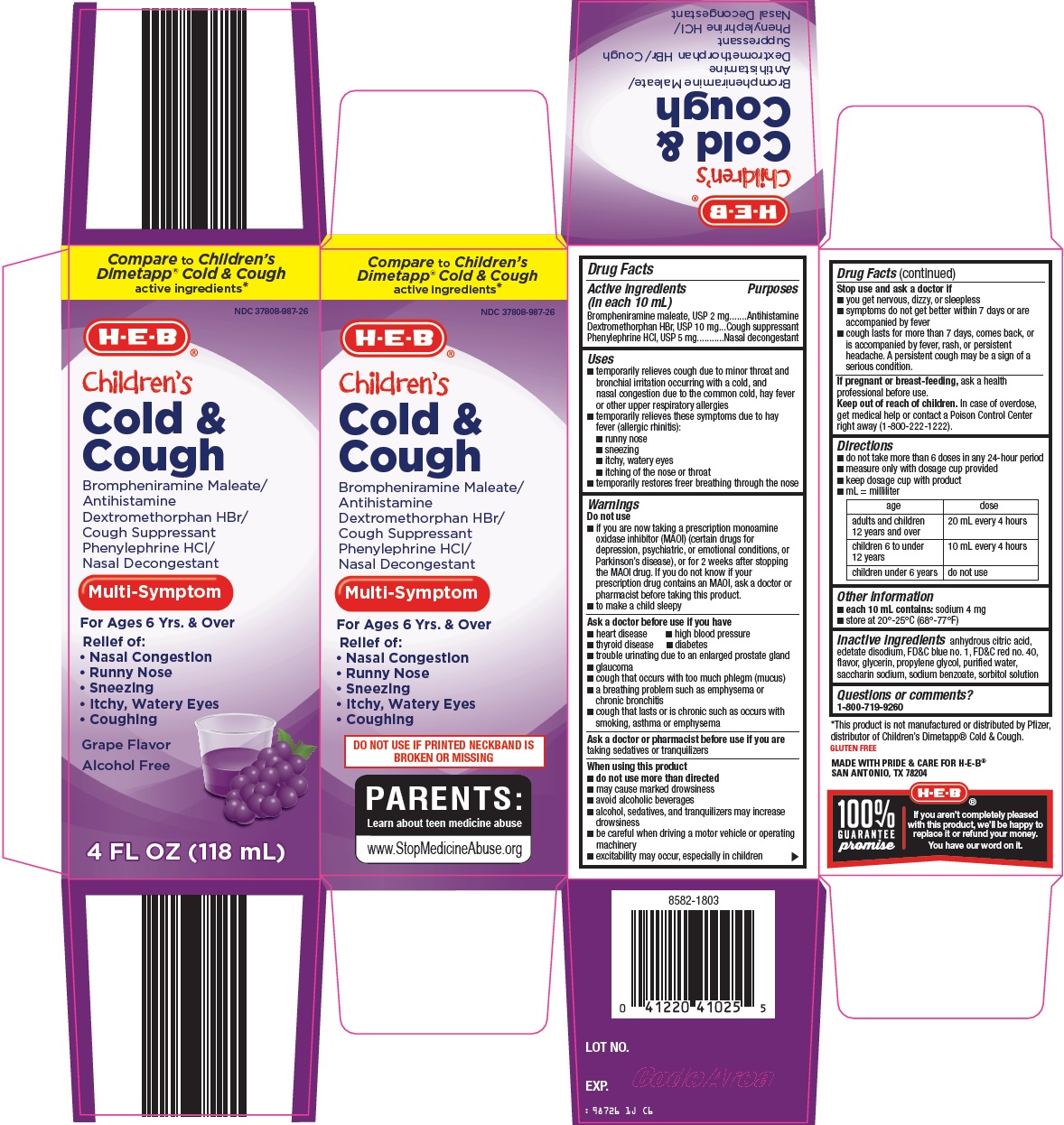
Label: COLD AND COUGH- brompheniramine maleate, dextromethorphan hbr, phenylephrine hcl solution
- NDC Code(s): 37808-987-26
- Packager: H E B
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each 10 mL)
- Purposes
-
Uses
- •
- temporarily relieves cough due to minor throat and bronchial irritation occurring with a cold, and nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- •
- temporarily relieves these symptoms due to hay fever (allergic rhinitis):
- •
- runny nose
- •
- sneezing
- •
- itchy, watery eyes
- •
- itching of the nose or throat
- •
- temporarily restores freer breathing through the nose
- Warnings
-
Do not use
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- •
- to make a child sleepy
-
Ask a doctor before use if you have
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to an enlarged prostate gland
- •
- glaucoma
- •
- cough that occurs with too much phlegm (mucus)
- •
- a breathing problem such as emphysema or chronic bronchitis
- •
- cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
- Ask a doctor or pharmacist before use if you are
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
Compare to Children’s Dimetapp® Cold & Cough active ingredients
children’s Cold & Cough
Brompheniramine Maleate/Antihistamine
Dextromethorphan HBr/Cough Suppressant
Phenylephrine HCl/Nasal Decongestant
Multi – Symptom
For Ages 6 Yrs. & Over
Relief of:
Nasal Congestion
Runny Nose
Sneezing
Itchy, Watery Eyes
Coughing
Grape Flavor
Alcohol Free
4 FL OZ (118 mL)
-
INGREDIENTS AND APPEARANCE
COLD AND COUGH
brompheniramine maleate, dextromethorphan hbr, phenylephrine hcl solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37808-987 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BROMPHENIRAMINE MALEATE (UNII: IXA7C9ZN03) (BROMPHENIRAMINE - UNII:H57G17P2FN) BROMPHENIRAMINE MALEATE 2 mg in 10 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg in 10 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg in 10 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) Product Characteristics Color PURPLE (Clear bluish-red) Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37808-987-26 1 in 1 CARTON 09/21/2006 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 09/21/2006 Labeler - H E B (007924756)