Label: LEVAMED SOLUBLE PIG WORMER- levamisole hydrochloride powder
- NDC Code(s): 61133-0280-1
- Packager: Bimeda, Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
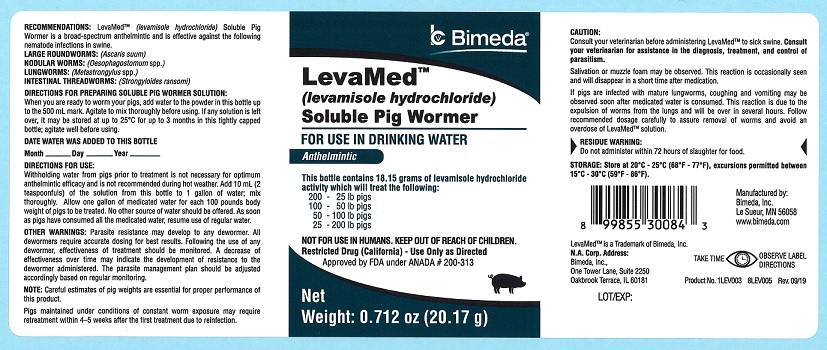
LevaMedTM
(levamisole hydrochloride)
Soluble Pig Wormer
FOR USE IN DRINKING WATER
Anthelmintic
This bottle contains 18.15 grams of levamisole hydrochloride activity which will treat the following:
200 - 25 lb pigs
100 - 50 lb pigs
50 - 100 lb pigs
25 - 200 lb pigs
NOT FOR USE IN HUMANS. KEEP OUT OF REACH OF CHILDREN.
Restricted Drug (California) - Use Only as Directed
Approved by FDA under ANADA # 200-313
-
SPL UNCLASSIFIED SECTION
RECOMMENDATIONS: LevaMed™ (levamisole hydrochloride) Soluble Pig Wormer is a broad-spectrum anthelmintic and is effective against the following nematode infections in swine.
LARGE ROUNDWORMS: (Ascaris suum)
NODULAR WORMS: (Oesophagostomum spp.)
LUNGWORMS: (Metastrongylus spp.)
INTESTINAL THREADWORMS: (Strongyloides ransomi)
-
DOSAGE & ADMINISTRATION
DIRECTIONS FOT PREPARING SOLUBLE PIG WORMER SOLUTION:
When you are ready to worm your pigs, add water to the powder in this bottle up to the 500 ml mark. Agitate to mix thoroughly before using. If any solution is left over, it may be stored at up to 25°C for up to 3 months in this tightly capped bottle; agitate well before using.
DATE WATER WAS ADDED TO THIS BOTTLE
Month ____ Day ____ Year ____
DIRECTIONS FOR USE:
Withholding water from pigs prior to treatment is not necessary for optimum anthelmintic efficacy and is not recommended during hot weather. Add 10 ml (2 teaspoonfuls) of the solution from this bottle to 1 gallon of water; mix thoroughly. Allow one gallon of medicated water for each 100 pounds body weight of pigs to be treated. No other source of water should be offered. As soon as pigs have consumed all the medicated water, resume use of regular water.
-
WARNINGS
OTHER WARNINGS: Parasite resistance may develop to any dewormer. All dewormers require accurate dosing for best results. Following the use of any dewormer, effectiveness of treatment should be monitored. A decrease of effectiveness over time may indicate the development of resistance to the dewormer administered. The parasite management plan should be adjusted accordingly based on regular monitoring.
NOTE: Careful estimates of pig weights are essential for proper performance of this product.
Pigs maintained under conditions of constant worm exposure may require retreatment within 4-5 weeks after the first treatment due to reinfection.
-
PRECAUTIONS
CAUTION:
Consult your veterinarian before administering LevaMed™ to sick swine. Consult your veterinarian for assistance in the diagnosis, treatment, and control of parasitism.
Salivation or muzzle foam may be observed. This reaction is occasionally seen and will disappear in a short time after medication.
If pigs are infected with mature lungworms, coughing and vomiting may be observed soon after medicated water is consumed. This reaction is due to the expulsion of worms from the lungs and will be over in several hours. Follow recommended dosage carefully to assure removal of worms and avoid an overdose of LevaMed™ solution.
- RESIDUE WARNING
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LEVAMED SOLUBLE PIG WORMER
levamisole hydrochloride powderProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:61133-0280 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVAMISOLE HYDROCHLORIDE (UNII: DL9055K809) (LEVAMISOLE - UNII:2880D3468G) LEVAMISOLE 20.17 g in 20.17 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61133-0280-1 20.17 g in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200313 05/13/2015 Labeler - Bimeda, Inc. (060492923) Registrant - Bimeda, Inc. (060492923) Establishment Name Address ID/FEI Business Operations Bimeda, Inc. 060492923 manufacture