Medication Guide
-
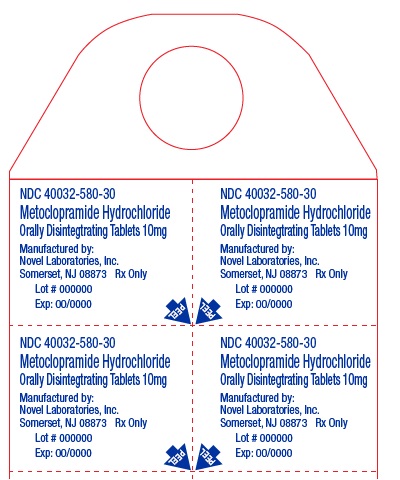
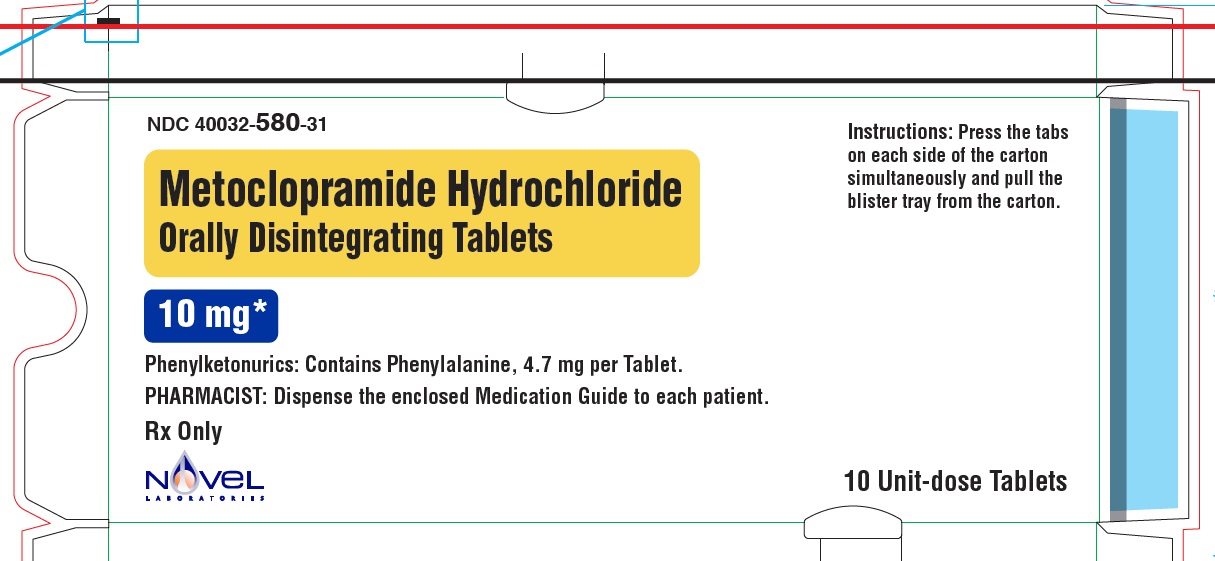
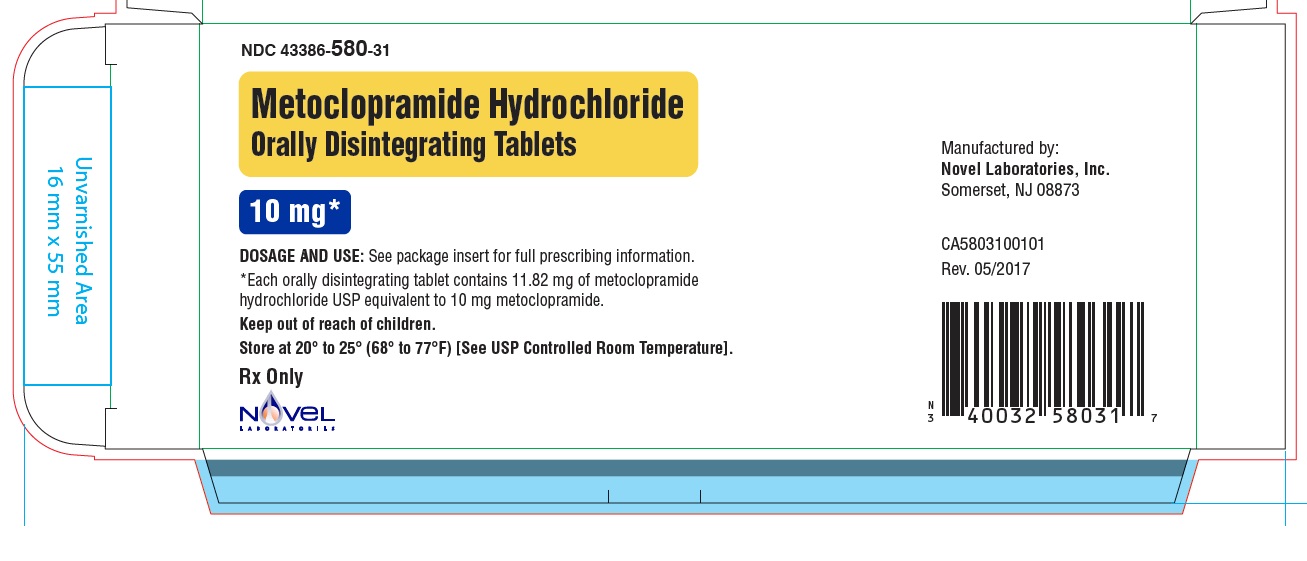
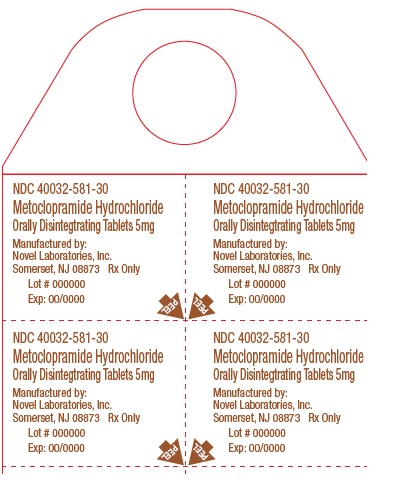
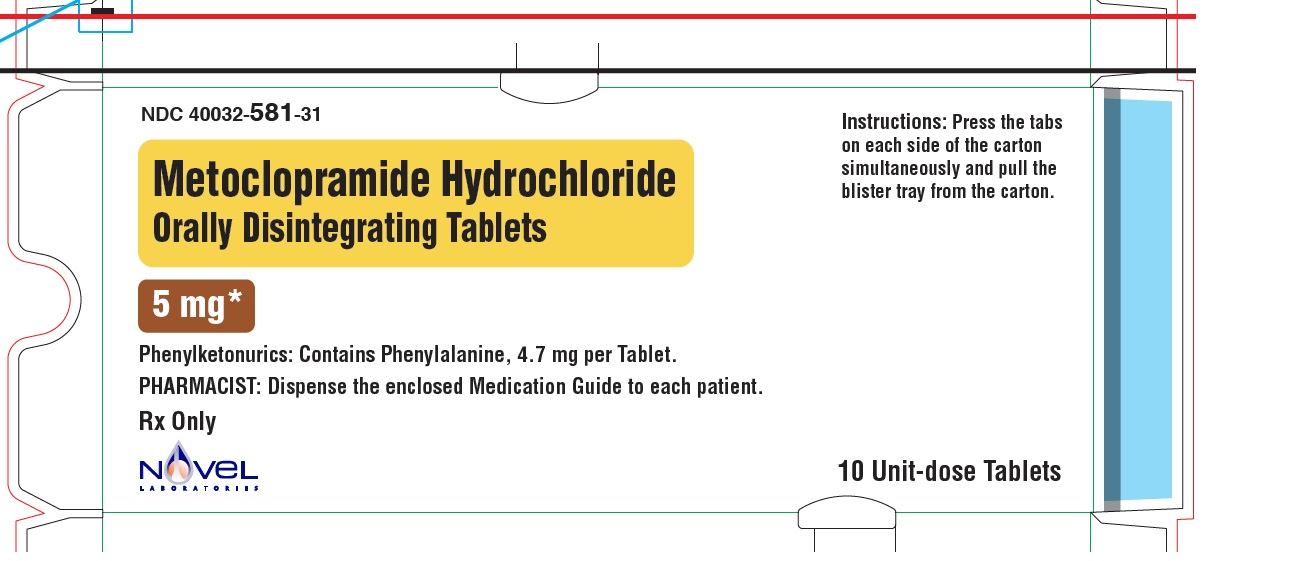
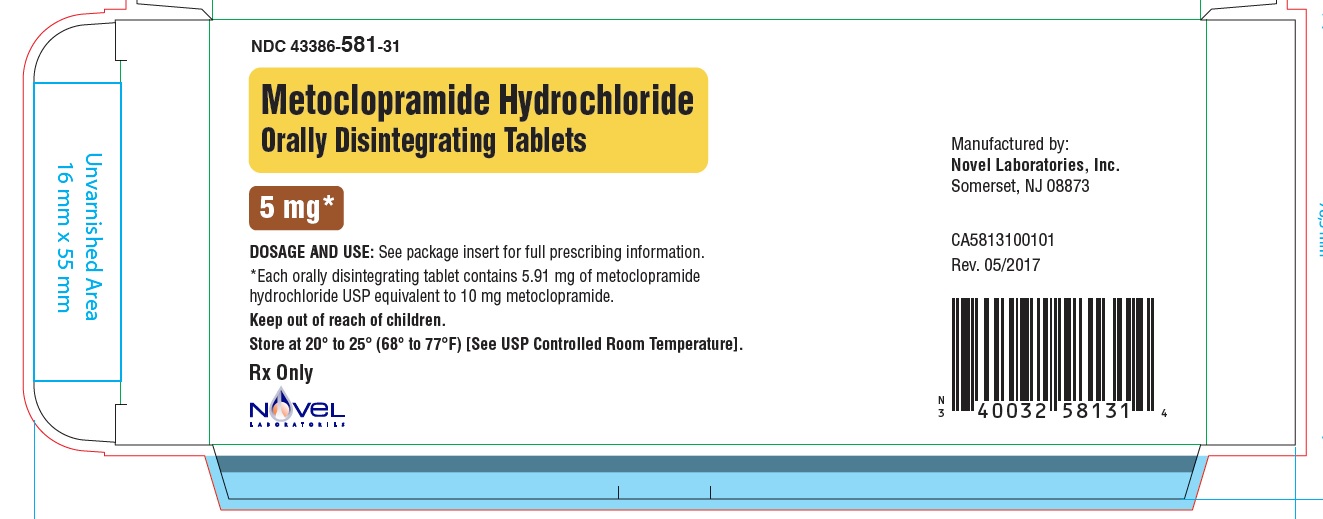
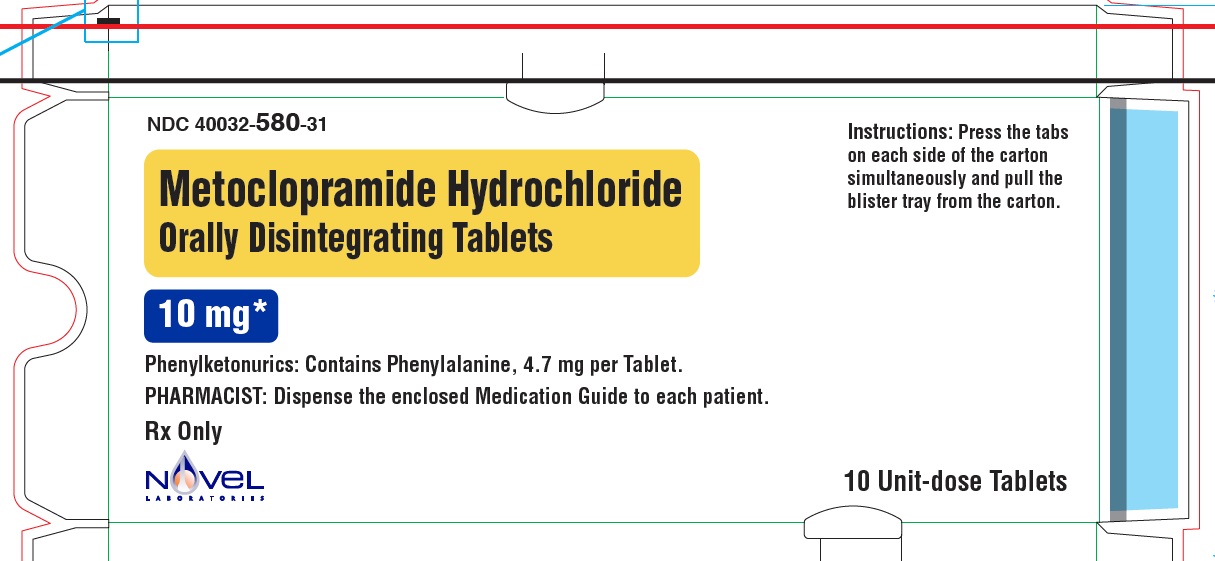
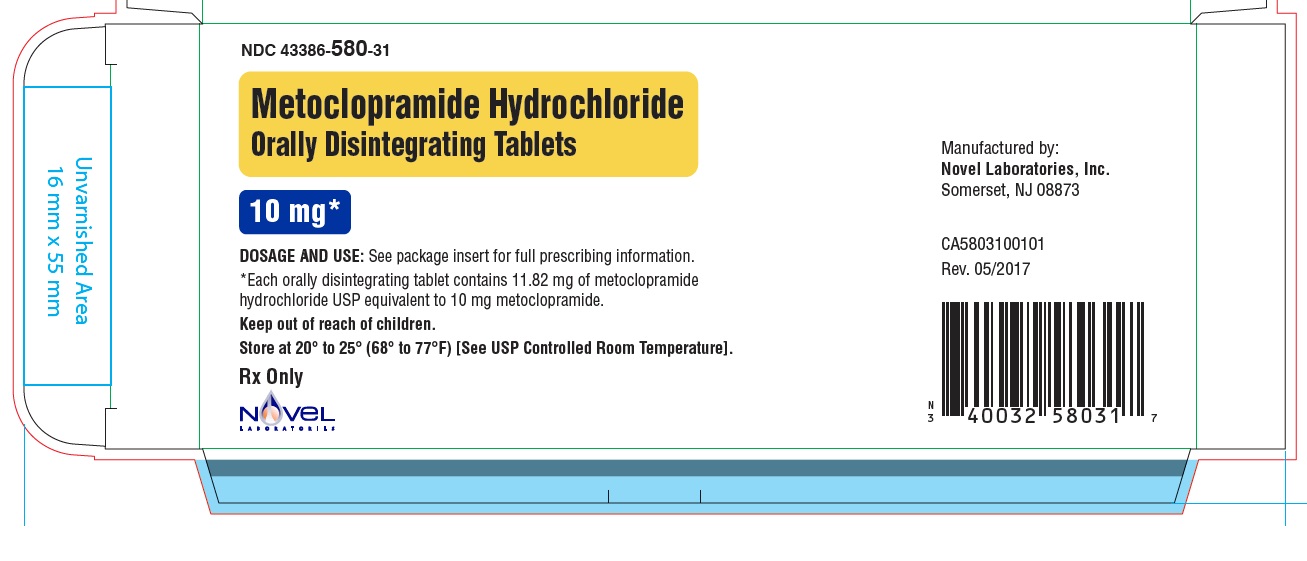
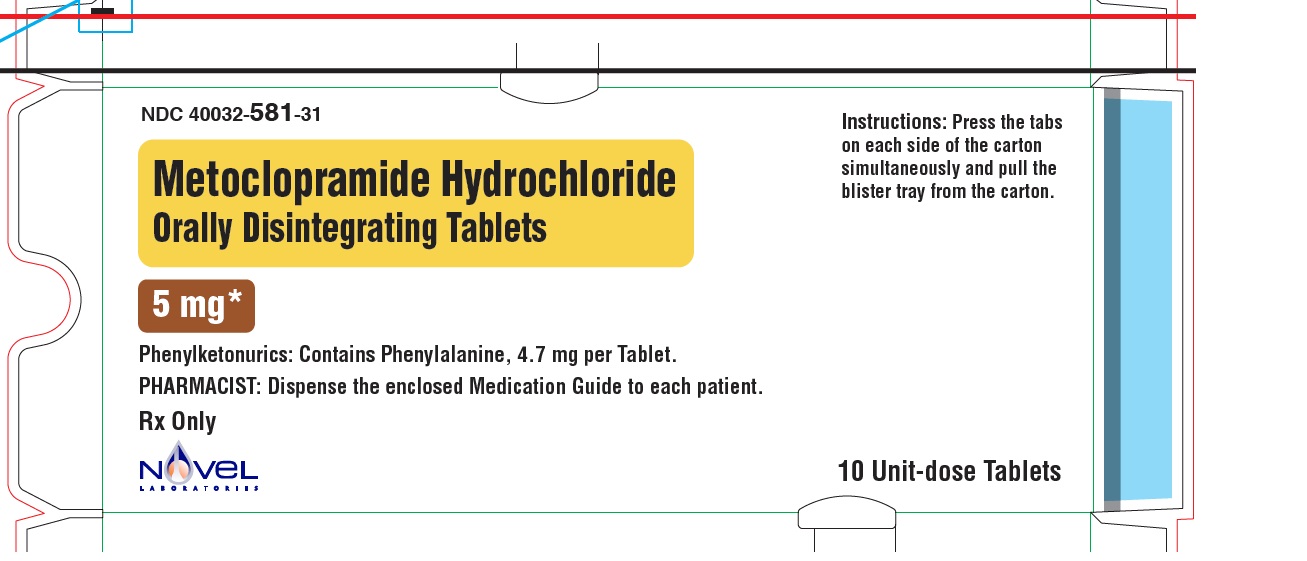
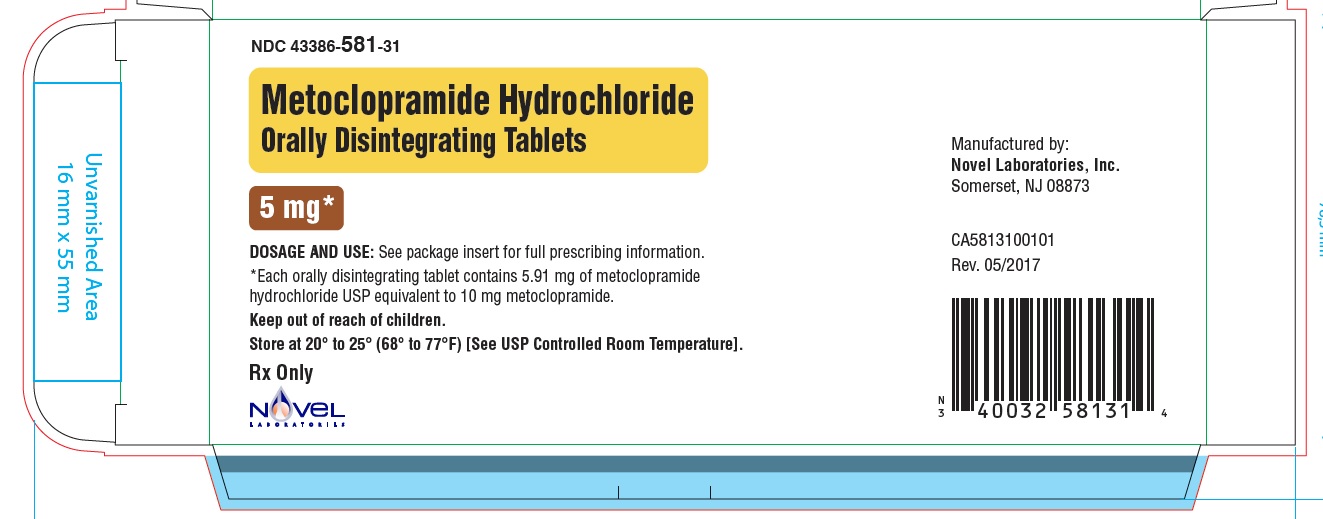
Metoclopramide Hydrochloride Orally Disintegrating Tablets
-
Phenylketonurics:
Phenylalanine is a component of aspartame. Each 5 mg and 10 mg Metoclopramide Hydrochloride ...
Medication Guide
Metoclopramide Hydrochloride Orally Disintegrating Tablets
Phenylketonurics:
Phenylalanine is a component of aspartame. Each 5 mg and 10 mg Metoclopramide Hydrochloride Orally Disintegrating Tablets contains 4.7 mg of phenylalanine.
Read the Medication Guide that comes with Metoclopramide Hydrochloride Orally Disintegrating Tablets before you take it and each time you get a refill. There may be new information. If you take another product that contains metoclopramide (such as REGLAN tablets, REGLAN ODT, REGLAN injection or metoclopramide oral solution), you should read the Medication Guide that comes with that product. Some of the information may be different. This Medication Guide does not take the place of talking with your doctor about your medical condition or your treatment.
What is the most important information I should know about Metoclopramide Hydrochloride Orally Disintegrating Tablets?
Metoclopramide Hydrochloride Orally Disintegrating Tablets can cause serious side effects, including:
Tardive Dyskinesia (abnormal muscle movements) These movements happen mostly in the face muscles. You cannot control these movements. They may not go away even after stopping Metoclopramide Hydrochloride Orally Disintegrating Tablets. There is no treatment for tardive dyskinesia, but symptoms may lessen or go away over time after you stop taking Metoclopramide Hydrochloride Orally Disintegrating Tablets.
Your chances for getting tardive dyskinesia go up:
- the longer you take Metoclopramide Hydrochloride Orally Disintegrating Tablets and the more Metoclopramide Hydrochloride Orally Disintegrating Tablets you take. You should not take Metoclopramide Hydrochloride Orally Disintegrating Tablets for more than 12 weeks.
- if you are older, especially if you are an older woman
- if you have diabetes
It is not possible for your doctor to know if you will get tardive dyskinesia if you take Metoclopramide Hydrochloride Orally Disintegrating Tablets.
Call your doctor right away if you have movements you can not stop or control, such as:
- lip smacking, chewing, or puckering of your lips
- frowning or scowling
- sticking out your tongue
- blinking and moving your eyes
- shaking of your arms and legs
See the section "What are the possible side effects of Metoclopramide Hydrochloride Orally Disintegrating Tablets?" for more information about side effects.
What is Metoclopramide Hydrochloride Orally Disintegrating Tablets?
Metoclopramide Hydrochloride Orally Disintegrating Tablets is a prescription medicine used in adults:
- for 4 to 12 weeks to relieve heartburn symptoms of gastroesophageal reflux disease (GERD) when certain other treatments do not work.
- to relieve the symptoms of slow stomach emptying in people with diabetes.
It is not known if Metoclopramide Hydrochloride Orally Disintegrating Tablets is safe or works in children.
Who should not take Metoclopramide Hydrochloride Orally Disintegrating Tablets?
Do not take Metoclopramide Hydrochloride Orally Disintegrating Tablets if you:
- have stomach or intestine problems that could get worse with Metoclopramide Hydrochloride Orally Disintegrating Tablets, such as bleeding, blockage or a tear in your stomach or bowel wall
- have an adrenal tumor called pheochromocytoma
- are allergic to metoclopramide or any of the ingredients in Metoclopramide Hydrochloride Orally Disintegrating Tablets. See the end of this Medication Guide for a list of ingredients in Metoclopramide Hydrochloride Orally Disintegrating Tablets.
- take medicines that can cause uncontrolled movements, such as medicines for mental illness.
- have seizures
What should I tell my doctor before taking Metoclopramide Hydrochloride Orally Disintegrating Tablets?
Before you take Metoclopramide Hydrochloride Orally Disintegrating Tablets, tell your doctor if you:
- have kidney or liver disease
- have depression or mental illness
- have high blood pressure
- have heart failure or heart rhythm problems
- have diabetes. Your dose of insulin may need to be changed.
- have Parkinson's disease
- have any other medical conditions
- drink alcohol
- are pregnant or plan to become pregnant. It is not known if Metoclopramide Hydrochloride Orally Disintegrating Tablets will harm your unborn baby.
- are breast-feeding or plan to breast-feed. Metoclopramide Hydrochloride Orally Disintegrating Tablets can pass into your milk and may harm your baby. You and your doctor should decide if you will take Metoclopramide Hydrochloride Orally Disintegrating Tablets or breast-feed. You should not do both.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Metoclopramide Hydrochloride Orally Disintegrating Tablets and some medicines can affect each other and may not work as well, or cause possible side effects. Do not start any new medicine while taking Metoclopramide Hydrochloride Orally Disintegrating Tablets until you talk with your doctor.
Especially tell your doctor if you take:
- another medicine that contains metoclopramide, such as REGLAN tablets, REGLAN ODT, or metoclopramide oral syrup
- a blood pressure medicine
- a medicine for depression, especially a monoamine oxidase inhibitor (MAOI)
- an anti-psychotic medicine
- insulin
- medicines that can make you sleepy, such as anti-anxiety medicines, sleep medicines, and narcotics.
Ask your doctor or pharmacist if you are not sure if your medication is listed above. Know the medicines you take. Keep a list of your medicines to show your doctor and pharmacist when you get new medicine.
How should I take Metoclopramide Hydrochloride Orally Disintegrating Tablets?
- Metoclopramide Hydrochloride Orally Disintegrating Tablets comes as a tablet that melts in your mouth.
- Take Metoclopramide Hydrochloride Orally Disintegrating Tablets exactly as prescribed by your doctor. Do not change your dose unless your doctor tells you to.
- You should not take Metoclopramide Hydrochloride Orally Disintegrating Tablets for more than 12 weeks.
- Take Metoclopramide Hydrochloride Orally Disintegrating Tablets at least 30 minutes before eating and at bedtime.
To take Metoclopramide Hydrochloride Orally Disintegrating Tablets:
- Leave the tablet in the sealed blister Metoclopramide Hydrochloride Orally Disintegrating Tablets pack until you are ready to take it.
- Use dry hands to open a blister and take out a tablet. If the tablet breaks or crumbles throw it away and take a new tablet out of the blister pack.
- Put the tablet on your tongue right away. Let it melt and then swallow. You do not need water to take Metoclopramide Hydrochloride Orally Disintegrating Tablets.
If you take too much Metoclopramide Hydrochloride Orally Disintegrating Tablets, call your doctor or Poison Control Center.
What should I avoid while taking Metoclopramide Hydrochloride Orally Disintegrating Tablets?
- Do not drink alcohol while taking Metoclopramide Hydrochloride Orally Disintegrating Tablets. Alcohol may make some side effects of Metoclopramide Hydrochloride Orally Disintegrating Tablets worse, such as feeling sleepy.
- Do not drive, work with machines, or do dangerous tasks until you know how Metoclopramide Hydrochloride Orally Disintegrating Tablets affects you. Metoclopramide Hydrochloride Orally Disintegrating Tablets may cause sleepiness.
What are the possible side effects of Metoclopramide Hydrochloride Orally Disintegrating Tablets?
Metoclopramide Hydrochloride Orally Disintegrating Tablets can cause serious side effects, including:
-
Tardivedyskinesia (abnormal muscle movements) See "What is the most important information I should know about Metoclopramide Hydrochloride Orally Disintegrating Tablets?"
-
Uncontrolled spasms of your face and neck muscles, or muscles of your body, arms, and legs (dystonia). These muscle spasms can cause abnormal movements and body positions. These spasms usually start within the first 2 days of treatment. These spasms happen more often in children and adults younger than 30.
-
Depression, thoughts about suicide, and suicide. Some people who take Metoclopramide Hydrochloride Orally Disintegrating Tablets may become depressed. You may have thoughts about hurting or killing yourself. Some people who have taken metoclopramide products have ended their own lives (suicide).
-
Neuroleptic Malignant Syndrome (NMS). NMS is a rare but very serious condition that can happen with Metoclopramide Hydrochloride Orally Disintegrating Tablets. NMS can cause death and must be treated in a hospital. Symptoms of NMS include: high fever, stiff muscles, problems thinking, very fast or uneven heartbeat, and increased sweating.
-
Parkinsonism. Symptoms include slight shaking, body stiffness, trouble moving or keeping your balance. If you have Parkinson's Disease, your symptoms may become worse while you are taking Metoclopramide Hydrochloride Orally Disintegrating Tablets.
-
High blood pressure. Metoclopramide Hydrochloride Orally Disintegrating Tablets can cause your blood pressure to increase.
-
Too much body water. People who have certain liver problems or heart failure and take Metoclopramide Hydrochloride Orally Disintegrating Tablets may hold too much water in their body (fluid retention). Tell your doctor right away if you have sudden weight gain, or swelling of your hands, legs, or feet.
Call your doctor and get medical help right away if you:
- feel depressed or have thoughts about hurting or killing yourself
- have high fever, stiff muscles, problems thinking, very fast or uneven heartbeat, and increased sweating
- have muscle movements you cannot stop or control
- have muscle movements that are new or unusual
The most common side effects of Metoclopramide Hydrochloride Orally Disintegrating Tablets are:
- headache
- nausea
- vomiting
- tiredness
- sleepiness
You may have more side effects the longer you take Metoclopramide Hydrochloride Orally Disintegrating Tablets and the more Metoclopramide Hydrochloride Orally Disintegrating Tablets you take.
You may still have side effects after you stop Metoclopramide Hydrochloride Orally Disintegrating Tablets. You may have symptoms from stopping (withdrawal) Metoclopramide Hydrochloride Orally Disintegrating Tablets such as headaches, and feeling dizzy or nervous.
Tell your doctor about any side effects that bother you or do not go away. These are not all the possible side effects of Metoclopramide Hydrochloride Orally Disintegrating Tablets.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1–800–FDA-1088.
How do I store Metoclopramide Hydrochloride Orally Disintegrating Tablets?
- Store Metoclopramide Hydrochloride Orally Disintegrating Tablets at room temperature, between 68°F to 77°F (20°C to 25°C).
- Keep Metoclopramide Hydrochloride Orally Disintegrating Tablets away from moisture.
- Throw away any Metoclopramide Hydrochloride Orally Disintegrating Tablets that is not used.
Keep Metoclopramide Hydrochloride Orally Disintegrating Tablets and all medicines away from children.
General information about Metoclopramide Hydrochloride Orally Disintegrating Tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Metoclopramide Hydrochloride Orally Disintegrating Tablets for a condition for which it was not prescribed. Do not give Metoclopramide Hydrochloride Orally Disintegrating Tablets to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about Metoclopramide Hydrochloride Orally Disintegrating Tablets. If you would like more information about Metoclopramide Hydrochloride Orally Disintegrating Tablets, talk with your doctor. You can ask your doctor or pharmacist for information about Metoclopramide Hydrochloride Orally Disintegrating Tablets that is written for health professionals.
What are the ingredients in Metoclopramide Hydrochloride Orally Disintegrating Tablets?
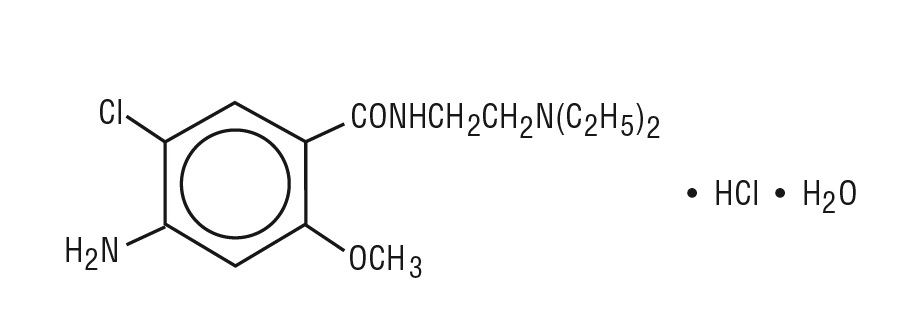
Active ingredients: metoclopramide hydrochloride
Inactive ingredients: phosphoric acid, mannitol and starch, microcrystalline cellulose, colloidal silicon dioxide, amino methacrylate copolymer, butylated hydroxyanisole, butylated hydroxytoluene, crospovidone, aspartame, N-C mint flavor, magnesium stearate.
Novel Laboratories, Inc
Somerset, NJ 08873
USA
PI5800000102
Rev 05/2017
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Close