Label: CUROXEN- olive extract, calendula ointment
- NDC Code(s): 71042-001-01, 71042-001-14, 71042-001-28
- Packager: OrganiCare Nature's Science, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- SPL UNCLASSIFIED SECTION
-
SPL UNCLASSIFIED SECTION
When we were kids, a sympathetic hug from mom and a dab of antibiotic ointment is how we treated minor cuts, scrapes, and burns. We still appreciate a hug, but treating wounds with most products on the market today means exposure to synthetic antiobiotics, which don't kill bacteria very well.
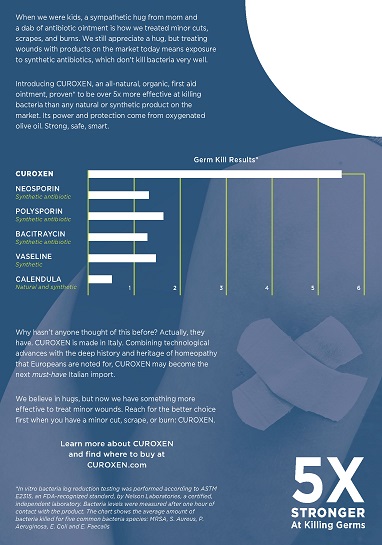
Introducing CUROXEN, an all-natural, organic first aid ointment, proven* to be over 5x more effective at killing bacteria than any other natural or synthetic product on the market. Its power and protection come from oxygened olive oil. Strong, safe, smart.
[CHART]
Why hasn't anyone thought of this before? Actually they have. CUROXEN is made in Italy. Combining technological advances with the deep history and heritage of homeopathy that Europeans are noted for, CUROXEN may become the next must-have Italian import.
We believe in hugs, but now we have something more effective to treat minor wounds. Reach for the better choice first when you have a minor cut, scrape, or burn: CUROXEN.
* In vitro bacteria log reduction testing was performed according to ASTM E2315, and FDA-recognized standard, by Nelson Laboratories, a certified, independent laboratory. Bacteria levels were measured after one hour of contact with the product. The chart shows the average amount of bacteria killed for five common bacteria species: MRSA, S. Aureus, P. Aeroginosa, E. Coli and E. Faecalis.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CUROXEN
olive extract, calendula ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71042-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLEA EUROPAEA FRUIT VOLATILE OIL (UNII: 8E7358CX1J) (OLEA EUROPAEA FRUIT VOLATILE OIL - UNII:8E7358CX1J) OLEA EUROPAEA FRUIT VOLATILE OIL 2 [hp_X] in 14 g CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 3 [hp_X] in 14 g Inactive Ingredients Ingredient Name Strength LAVENDER OIL (UNII: ZBP1YXW0H8) OLIVE OIL (UNII: 6UYK2W1W1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71042-001-14 1 in 1 CARTON 10/21/2016 1 14 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:71042-001-01 1 in 1 BLISTER PACK 01/19/2017 2 1 g in 1 POUCH; Type 0: Not a Combination Product 3 NDC:71042-001-28 1 in 1 CARTON 12/20/2017 3 28.3 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/21/2016 Labeler - OrganiCare Nature's Science, LLC (044204745)




