Label: GLYCOPYRROLATE solution
- NDC Code(s): 71656-061-16
- Packager: Saptalis Pharmaceuticals, LLC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GLYCOPYRROLATE ORAL SOLUTION safely and effectively. See full prescribing information for GLYCOPYRROLATE ORAL SOLUTION ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEGlycopyrrolate oral solution is indicated to reduce chronic severe drooling in patients aged 3 years to 16 years with neurologic conditions associated with problem drooling (e.g., cerebral ...
-
2 DOSAGE AND ADMINISTRATIONGlycopyrrolate oral solution must be measured and administered with an accurate measuring device [see - Patient Counseling Information (17)]. Initiate dosing at 0.02 mg/kg orally three times ...
-
3 DOSAGE FORMS AND STRENGTHSGlycopyrrolate oral solution is available as a 1 mg/5 mL clear, transparent liquid, free of visible particulate matter for oral administration in 16 fl. oz. (473 mL) bottles.
-
4 CONTRAINDICATIONSGlycopyrrolate oral solution is contraindicated in: Patients with medical conditions that preclude anticholinergic therapy (e.g., glaucoma, paralytic ileus, unstable cardiovascular status in ...
-
5 WARNINGS AND PRECAUTIONS5.1 Constipation or Intestinal Pseudo-obstruction - Constipation is a common dose-limiting adverse reaction which sometimes leads to glycopyrrolate discontinuation [see - Adverse Reactions ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described elsewhere in the labeling: Constipation or intestinal pseudo-obstruction [see - Warnings and Precautions (5.1)] Incomplete ...
-
7 DRUG INTERACTIONSDrugs Affected by Reduced GI Transit Time - Glycopyrrolate reduces GI transit time, which may result in altered release of certain drugs when formulated in delayed- or controlled-release dosage ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data in pregnant women for glycopyrrolate oral solution to inform decisions concerning any drug-associated risks. In pregnant rats, daily ...
-
10 OVERDOSAGEBecause glycopyrrolate is a quaternary amine which does not easily cross the blood-brain barrier, symptoms of glycopyrrolate overdosage are generally more peripheral in nature rather than central ...
-
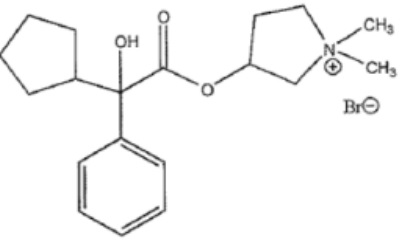
11 DESCRIPTIONGlycopyrrolate is an anticholinergic drug available as an oral solution containing 1 mg glycopyrrolate, USP per 5 mL. The chemical name for glycopyrrolate is pyrrolidinium ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Glycopyrrolate is a competitive inhibitor of acetylcholine receptors that are located on certain peripheral tissues, including salivary glands. Glycopyrrolate ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - When glycopyrrolate was administered via oral gavage to mice for up to 24 months at dosages of 2.5 mg/kg/day, 7 mg/kg/day, and 20 ...
-
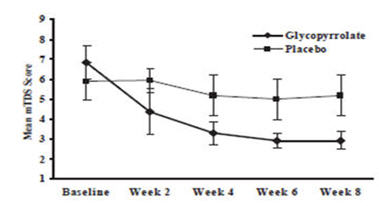
14 CLINICAL STUDIESGlycopyrrolate oral solution was evaluated in a multi-center, randomized, double-blind, placebo-controlled, parallel, eight-week study for the control of pathologic drooling in children (Study 1) ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGGlycopyrrolate oral solution 1 mg/5 mL is a clear, transparent liquid, free of visible particulate matter supplied as follows: NDC 71656-061-16 16 fl. oz. (473 mL) Bottle with ...
-
17 PATIENT COUNSELING INFORMATIONSee - FDA-approved patient labeling (Patient Information) Advise patients/caregivers to measure glycopyrrolate oral solution with an accurate measuring device. A household teaspoon is not an ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Saptalis Pharmaceuticals, LLC. Hauppauge, NY 11788 - September 2024-R3 - PPM-0044
-
PATIENT PACKAGE INSERTPATIENT and CAREGIVER INFORMATION - Glycopyrrolate - (glye-koe-pye-roe-late) Oral Solution - Please read the Patient and Caregiver Information that comes with glycopyrrolate oral solution before ...
-
PRINCIPAL DISPLAY PANEL - 473 mL Bottle LabelNDC 71656-061-16 - Glycopyrrolate Oral Solution - 1 mg/5 mL - (0.2 mg/mL) For Oral Use Only - Rx only - 16 fl. oz. (473 mL)
-
INGREDIENTS AND APPEARANCEProduct Information