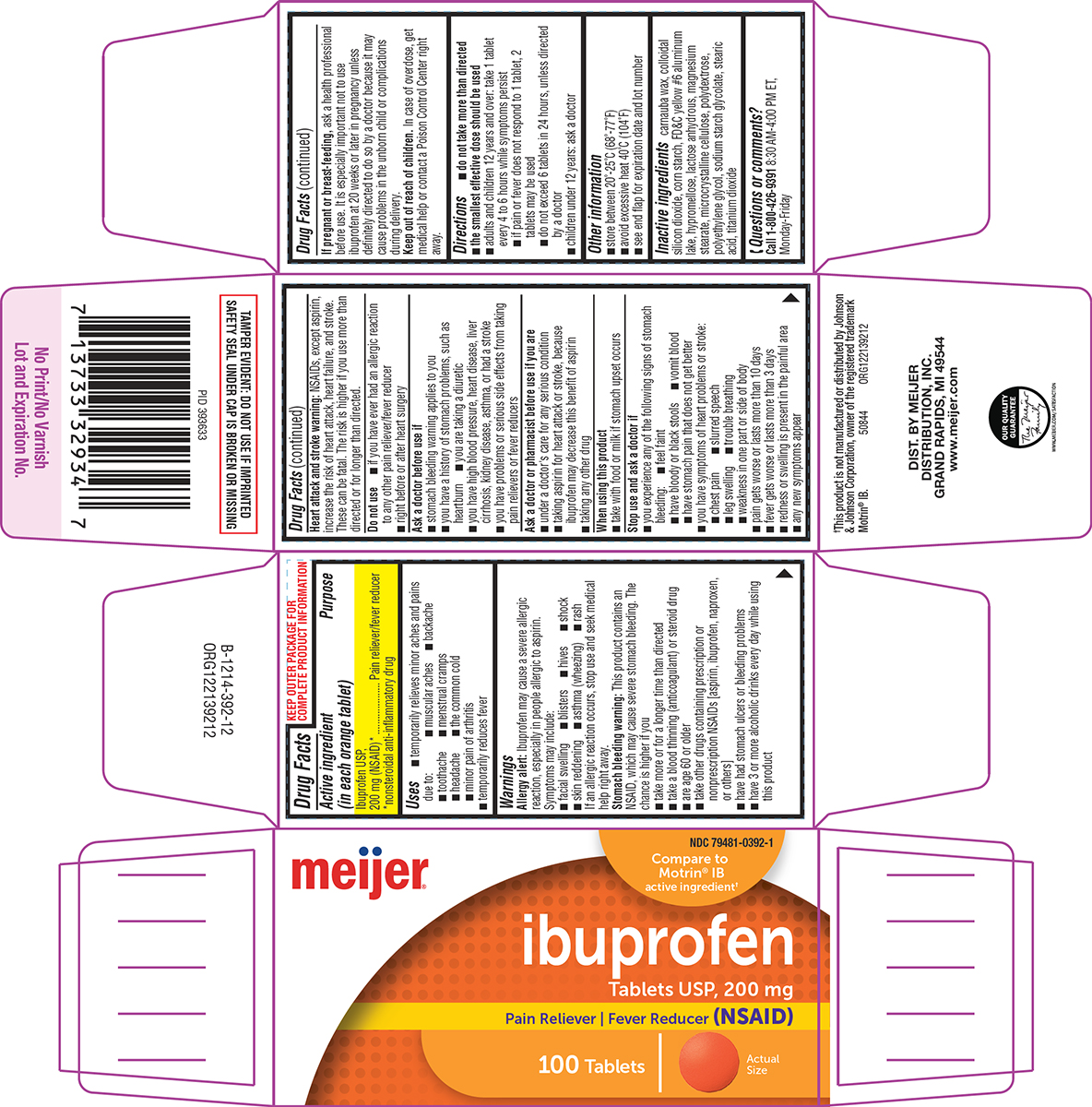
Label: IBUPROFEN tablet, film coated
- NDC Code(s): 79481-0392-1
- Packager: Meijer Distribution, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 14, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient (in each orange tablet)
Ibuprofen USP, 200 mg (NSAID)* *nonsteroidal anti-inflammatory drug
-
Purpose
Pain reliever/fever reducer
-
Uses
temporarily relieves minor aches and pains due to: muscular aches - backache - toothache - menstrual cramps - headache - the common cold - minor pain of arthritis - temporarily reduces fever
-
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include: facial swelling - blisters - hives - shock - skin reddening - asthma ...
-
Directions
do not take more than directed - the smallest effective dose should be used - adults and children 12 years and over: take 1 tablet every 4 to 6 hours while symptoms persist - if pain or fever ...
-
Other information
store between 20º-25ºC (68º-77ºF) avoid excessive heat 40ºC (104ºF) see end flap for expiration date and lot number
-
Inactive ingredients
carnauba wax, colloidal silicon dioxide, corn starch, FD&C yellow #6 aluminum lake, hypromellose, lactose anhydrous, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene ...
-
Questions or comments?
Call 1-800-426-9391 8:30 AM-4:00 PM ET, Monday-Friday
-
Principal Display Panel
meijer® NDC 79481-0392-1 - Compare to - Motrin® IB - active ingredient† ibuprofen - Tablets USP, 200 mg - Pain Reliever | Fever Reducer (NSAID) 100 Tablets - Actual - Size - TAMPER EVIDENT: DO NOT USE ...
-
INGREDIENTS AND APPEARANCEProduct Information