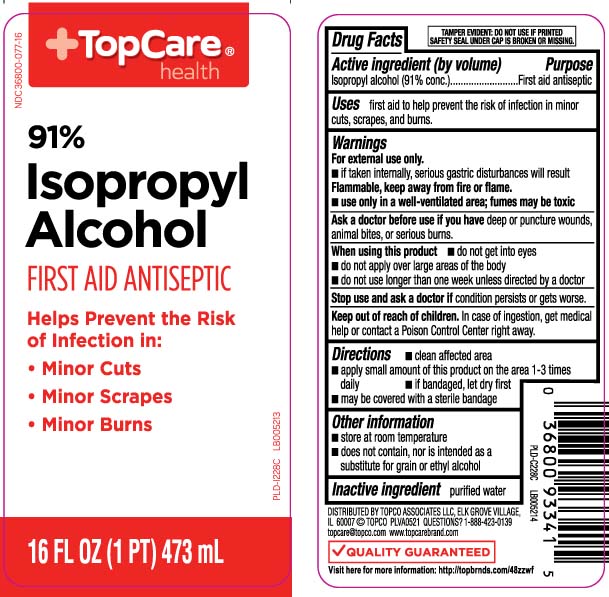
Label: ISOPROPYL ALCOHOL 91 PERCENT- isopropyl alcohol liquid
- NDC Code(s): 36800-077-16
- Packager: TOP CARE (Topco Associates LLC)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (by volume)
- Purpose
- Uses
-
Warnings
For external use only.
- if taken internally serious gastric disturbances will result
Flammable, keep away from fire or flame.
- use only in a well-ventilated area; fumes may be toxic
- Directions
- Other information
- Inactive ingredient
-
Principal Display Panel
91% Isopropyl Alcohol
FIRST AID ANTISEPTIC
Helps prevent the risk of infection in:
- Minor cuts
- Minor scrapes
- Minor burns
FL OZ (mL)
Question? 1-888-423-0139
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER THE CAP IS BROKEN OR MISSING.
DISTRIBUTED BY TOPCO ASSOCIATES LLC
ELK GROVE VILLAGE, IL 60007
- Package Label
-
INGREDIENTS AND APPEARANCE
ISOPROPYL ALCOHOL 91 PERCENT
isopropyl alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:36800-077 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 91 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:36800-077-16 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/31/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 10/31/2014 Labeler - TOP CARE (Topco Associates LLC) (006935977)