Label: GADAVIST- gadobutrol injection
-
NDC Code(s):
50419-325-11,
50419-325-12,
50419-325-13,
50419-325-27, view more50419-325-28, 50419-325-29, 50419-325-37, 50419-325-72, 50419-325-75
- Packager: Bayer HealthCare Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 5, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GADAVIST safely and effectively. See full prescribing information for GADAVIST - GADAVIST (gadobutrol) injection, for intravenous use ...These highlights do not include all the information needed to use GADAVIST safely and effectively. See full prescribing information for GADAVIST
GADAVIST (gadobutrol) injection, for intravenous use
Initial U.S. Approval: 2011WARNING: RISK ASSOCIATED WITH INTRATHECAL USE and NEPHROGENIC SYSTEMIC FIBROSIS
See full prescribing information for complete boxed warning
- •
- Intrathecal administration of gadolinium-based contrast agents (GBCAs) can cause serious adverse reactions including death, coma, encephalopathy, and seizures. Gadavist is not approved for intrathecal use (5.1)
- •
- GBCAs increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of Gadavist in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities.
- The risk for NSF appears highest among patients with:
- •
- Chronic, severe kidney disease (GFR < 30 mL/min/1.73m2), or
- •
- Acute kidney injury.
- Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (for example, age >60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing. (5.2)
RECENT MAJOR CHANGES
Warnings and Precautions, Acute Respiratory Distress Syndrome (5.4) 3/2025
INDICATIONS AND USAGE
Gadavist is a gadolinium-based contrast agent indicated for use with magnetic resonance imaging (MRI):
- •
- To detect and visualize areas with disrupted blood brain barrier and/or abnormal vascularity of the central nervous system in adult and pediatric patients, including term neonates (1.1)
- •
- To assess the presence and extent of malignant breast disease in adult patients (1.2)
- •
- To evaluate known or suspected supra-aortic or renal artery disease in adult and pediatric patients, including term neonates (1.3)
- •
- To assess myocardial perfusion (stress, rest) and late gadolinium enhancement in adult patients with known or suspected coronary artery disease (CAD). (1.4).
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Gadavist injection contains 604.72 mg gadobutrol/mL (equivalent to 1 mmol gadobutrol/mL) and is available in vials and prefilled syringes (3)
CONTRAINDICATIONS
History of severe hypersensitivity reaction to Gadavist (4)
WARNINGS AND PRECAUTIONS
- •
- Hypersensitivity Reactions: Anaphylactic and other hypersensitivity reactions with cardiovascular, respiratory or cutaneous manifestations, ranging from mild to severe, including death, have occurred. Monitor patients closely during and after administration of Gadavist. (5.3)
- •
- Acute Respiratory Distress Syndrome: For patients demonstrating respiratory distress after administration, assess oxygen requirement and monitor for worsening respiratory function. (5.4)
- •
- Gadolinium Retention: Gadolinium is retained for months or years in brain, bone, and other organs. (5.5)
ADVERSE REACTIONS
- •
- Most common adverse reactions (incidence ≥ 0.5%) are headache, nausea, and dizziness (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bayer HealthCare Pharmaceuticals Inc. at 1-888-842-2937 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
USE IN SPECIFIC POPULATIONS
Pregnancy: Use only if imaging is essential during pregnancy and cannot be delayed. (8.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 3/2025
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISK ASSOCIATED WITH INTRATHECAL USE and NEPHROGENIC SYSTEMIC FIBROSIS
1 INDICATIONS AND USAGE
1.1 Magnetic Resonance Imaging (MRI) of the Central Nervous System (CNS)
1.2 MRI of the Breast
1.3 Magnetic Resonance Angiography (MRA)
1.4 Cardiac MRI
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
2.2 Administration Guidelines
2.3 Drug Handling
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.2 Nephrogenic Systemic Fibrosis
5.3 Hypersensitivity Reactions
5.5 Gadolinium Retention
5.6 Acute Kidney Injury
5.7 Extravasation and Injection Site Reactions
5.8 Overestimation of Extent of Malignant Disease in MRI of the Breast
5.9 Low Sensitivity for Significant Arterial Stenosis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 MRI of the CNS
14.2 MRI of the Breast
14.3 MRA
14.4 Cardiac MRI
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISK ASSOCIATED WITH INTRATHECAL USE and NEPHROGENIC SYSTEMIC FIBROSIS
Risk Associated with Intrathecal use
Intrathecal administration of gadolinium-based contrast agents (GBCAs) can cause serious adverse reactions including death, coma, encephalopathy, and seizures. Gadavist is not approved for intrathecal use [see Warnings and Precautions (5.1)].
Nephrogenic Systemic Fibrosis
GBCAs increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of Gadavist in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs.
The risk for NSF appears highest among patients with:
- •
- Chronic, severe kidney disease (GFR < 30 mL/min/1.73m2), or
- •
- Acute kidney injury.
Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (for example, age > 60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing.
For patients at highest risk for NSF, do not exceed the recommended Gadavist dose and allow a sufficient period of time for elimination of the drug from the body prior to any re-administration [see Warnings and Precautions (5.2)].
Close -
1 INDICATIONS AND USAGE1.1 Magnetic Resonance Imaging (MRI) of the Central Nervous System (CNS) Gadavist is indicated for use with magnetic resonance imaging (MRI) in adult and pediatric patients, including term ...
1.1 Magnetic Resonance Imaging (MRI) of the Central Nervous System (CNS)
Gadavist is indicated for use with magnetic resonance imaging (MRI) in adult and pediatric patients, including term neonates, to detect and visualize areas with disrupted blood brain barrier and/or abnormal vascularity of the central nervous system.
1.2 MRI of the Breast
Gadavist is indicated for use with MRI in adult patients to assess the presence and extent of malignant breast disease.
1.3 Magnetic Resonance Angiography (MRA)
Gadavist is indicated for use in magnetic resonance angiography (MRA) in adult and pediatric patients, including term neonates, to evaluate known or suspected supra-aortic or renal artery disease.
Close1.4 Cardiac MRI
Gadavist is indicated for use in cardiac MRI (CMRI) to assess myocardial perfusion (stress, rest) and late gadolinium enhancement in adult patients with known or suspected coronary artery disease (CAD).
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dose - The recommended dose of Gadavist for adult and pediatric patients (including term neonates) is 0.1 mL/kg body weight (0.1 mmol/kg). Refer to Table 1 to determine the volume ...
2.1 Recommended Dose
The recommended dose of Gadavist for adult and pediatric patients (including term neonates) is 0.1 mL/kg body weight (0.1 mmol/kg). Refer to Table 1 to determine the volume to be administered.
Table 1: Volume of Gadavist Injection by Body Weight* Body Weight (kg)
Volume to be Administered (mL)
2.5
0.25
5
0.5
10
1
15
1.5
20
2
25
2.5
30
3
35
3.5
40
4
45
4.5
50
5
60
6
70
7
80
8
90
9
100
10
110
11
120
12
130
13
140
14
*for Cardiac MRI, the dose is divided into 2 separate, equal injections
2.2 Administration Guidelines
- •
- Gadavist is formulated at a higher concentration (1 mmol/mL) compared to certain other gadolinium based contrast agents, resulting in a lower volume of administration. Use Table 1 to determine the volume to be administered.
- •
- Use sterile technique when preparing and administering Gadavist.
MRI of the Central Nervous System
- •
- Administer Gadavist as an intravenous injection, manually or by power injector, at a flow rate of approximately 2 mL/second.
- •
- Follow Gadavist injection with a normal saline flush to ensure complete administration of the contrast.
- •
- Post contrast MRI can commence immediately following contrast administration.
MRI of the Breast
- •
- Administer Gadavist as an intravenous bolus by power injector, followed by a normal saline flush to ensure complete administration of the contrast.
- •
- Start image acquisition following contrast administration and then repeat sequentially to determine peak intensity and wash-out.
MR Angiography
Image acquisition should coincide with peak arterial concentration, which varies among patients.
Cardiac MRI
- •
- Administer Gadavist through a separate intravenous line in the contralateral arm if concomitantly providing a continuous infusion of a pharmacologic stress agent.
- •
- Administer Gadavist as two (2) separate bolus injections: 0.05 mL/kg (0.05 mmol/kg) body weight at peak pharmacologic stress followed by 0.05 mL/kg (0.05 mmol/kg) body weight at rest.
- •
- Administer Gadavist via a power injector at a flow rate of approximately 4 mL/second and follow each injection with a normal saline flush of 20 mL at the same flow rate.
Close2.3 Drug Handling
- •
- Visually inspect Gadavist for particulate matter and discoloration prior to administration. Do not use the solution if it is discolored, if particulate matter is present or if the container appears damaged.
- •
- Do not mix Gadavist with other medications and do not administer Gadavist in the same intravenous line simultaneously with other medications because of the potential for chemical incompatibility.
-
3 DOSAGE FORMS AND STRENGTHSGadavist is a sterile, clear, and colorless to pale yellow solution for injection containing 604.72 mg gadobutrol per mL (equivalent to 1 mmol gadobutrol/mL) supplied in single-dose vials and ...
Gadavist is a sterile, clear, and colorless to pale yellow solution for injection containing 604.72 mg gadobutrol per mL (equivalent to 1 mmol gadobutrol/mL) supplied in single-dose vials and pre-filled disposable syringes.
Close -
4 CONTRAINDICATIONSGadavist is contraindicated in patients with history of severe hypersensitivity reactions to Gadavist.
Gadavist is contraindicated in patients with history of severe hypersensitivity reactions to Gadavist.
Close -
5 WARNINGS AND PRECAUTIONSIntrathecal administration of GBCAs can cause serious adverse reactions including death, coma, encephalopathy, and seizures. The safety and effectiveness of Gadavist have not been established with ...
Intrathecal administration of GBCAs can cause serious adverse reactions including death, coma, encephalopathy, and seizures. The safety and effectiveness of Gadavist have not been established with intrathecal use. Gadavist is not approved for intrathecal use [see Dosage and Administration (2.2)].
5.2 Nephrogenic Systemic Fibrosis
GBCAs increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of Gadavist among these patients unless the diagnostic information is essential and not available with non-contrast MRI or other modalities. The GBCA-associated NSF risk appears highest for patients with chronic, severe kidney disease (GFR < 30 mL/min/1.73m2) as well as patients with acute kidney injury. The risk appears lower for patients with chronic, moderate kidney disease (GFR 30 to 59 mL/min/1.73m2) and little, if any, for patients with chronic, mild kidney disease (GFR 60 to 89 mL/min/1.73m2). NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs. Report any diagnosis of NSF following Gadavist administration to Bayer Healthcare (1-888-842-2937) or FDA (1-800-FDA-1088 or www.fda.gov/medwatch).
Screen patients for acute kidney injury and other conditions that may reduce renal function. Features of acute kidney injury consist of rapid (over hours to days) and usually reversible decrease in kidney function, commonly in the setting of surgery, severe infection, injury or drug-induced kidney toxicity. Serum creatinine levels and estimated GFR may not reliably assess renal function in the setting of acute kidney injury. For patients at risk for chronically reduced renal function (for example, age > 60 years, diabetes mellitus or chronic hypertension), estimate the GFR through laboratory testing.
Among the factors that may increase the risk for NSF are repeated or higher than recommended doses of a GBCA and degree of renal impairment at the time of exposure. Record the specific GBCA and the dose administered to a patient. For patients at highest risk for NSF, do not exceed the recommended Gadavist dose and allow a sufficient period of time for elimination of the drug prior to re-administration. For patients receiving hemodialysis, consider the prompt initiation of hemodialysis following the administration of a GBCA in order to enhance the contrast agent’s elimination [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]. The usefulness of hemodialysis in the prevention of NSF is unknown [see Clinical Pharmacology (12.3)].
5.3 Hypersensitivity Reactions
Anaphylactic and other hypersensitivity reactions with cardiovascular, respiratory or cutaneous manifestations, ranging from mild to severe, including death, have uncommonly occurred following Gadavist administration [see Adverse Reactions (6)].
- •
- Before Gadavist administration, assess all patients for any history of a reaction to contrast media, bronchial asthma and/or allergic disorders. These patients may have an increased risk for a hypersensitivity reaction to Gadavist.
- •
- Administer Gadavist only in situations where trained personnel and therapies are promptly available for the treatment of hypersensitivity reactions, including personnel trained in resuscitation.
Most hypersensitivity reactions to Gadavist have occurred within half an hour after administration. Delayed reactions can occur up to several days after administration. Observe patients for signs and symptoms of hypersensitivity reactions during and following Gadavist administration.
5.4 Acute Respiratory Distress Syndrome
Acute respiratory distress syndrome (ARDS) has been reported in patients administered GADAVIST and may be characterized by severe hypoxemia requiring oxygen support and mechanical ventilation. These manifestations may resemble an immediate hypersensitivity reaction with onset of respiratory distress within <30 minutes to 24 hours after GADAVIST administration. For patients demonstrating respiratory distress after GADAVIST administration, assess oxygen requirement and monitor for worsening respiratory function.
5.5 Gadolinium Retention
Gadolinium is retained for months or years in several organs. The highest concentrations (nanomoles per gram of tissue) have been identified in the bone, followed by other organs (for example, brain, skin, kidney, liver, and spleen). The duration of retention also varies by tissue and is longest in bone. Linear GBCAs cause more retention than macrocyclic GBCAs. At equivalent doses, gadolinium retention varies among the linear agents with Omniscan (gadodiamide) and Optimark (gadoversetamide) causing greater retention than other linear agents [Eovist (gadoxetate disodium), Magnevist (gadopentetate dimeglumine), MultiHance (gadobenate dimeglumine)]. Retention is lowest and similar among the macrocyclic GBCAs [Dotarem (gadoterate meglumine), Gadavist (gadobutrol), ProHance (gadoteridol)].
Consequences of gadolinium retention in the brain have not been established. Pathologic and clinical consequences of GBCA administration and retention in skin and other organs have been established in patients with impaired renal function [see Warnings and Precautions (5.2)]. There are rare reports of pathologic skin changes in patients with normal renal function. Adverse events involving multiple organ systems have been reported in patients with normal renal function without an established causal link to gadolinium retention [see Adverse Reactions (6.2)].
While clinical consequences of gadolinium retention have not been established in patients with normal renal function, certain patients might be at higher risk. These include patients requiring multiple lifetime doses, pregnant and pediatric patients, and patients with inflammatory conditions. Consider the retention characteristics of the agent when choosing a GBCA for these patients. Minimize repetitive GBCA imaging studies particularly closely spaced studies, when possible.
5.6 Acute Kidney Injury
In patients with chronic renal impairment, acute kidney injury sometimes requiring dialysis has been observed with the use of some GBCAs. Do not exceed the recommended dose; the risk of acute kidney injury may increase with higher than recommended doses.
5.7 Extravasation and Injection Site Reactions
Ensure catheter and venous patency before the injection of Gadavist. Extravasation into tissues during Gadavist administration may result in moderate irritation [see Nonclinical Toxicology (13.2)].
5.8 Overestimation of Extent of Malignant Disease in MRI of the Breast
Gadavist MRI of the breast overestimated the histologically confirmed extent of malignancy in the diseased breast in up to 50% of the patients [see Clinical Studies (14.2)].
Close5.9 Low Sensitivity for Significant Arterial Stenosis
The performance of Gadavist MRA for detecting arterial segments with significant stenosis (>50% renal, >70% supra-aortic) has not been shown to exceed 55%. Therefore, a negative MRA study alone should not be used to rule out significant stenosis [see Clinical Studies (14.3)].
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are discussed elsewhere in labeling: • Nephrogenic Systemic Fibrosis (NSF) [see Boxed Warning and Warnings and Precautions ...
- The following clinically significant adverse reactions are discussed elsewhere in labeling:
- •
- Nephrogenic Systemic Fibrosis (NSF) [see Boxed Warning and Warnings and Precautions (5.2)].
- •
- Hypersensitivity reactions [see Contraindications (4) and Warnings and Precautions (5.3)].
- •
- Acute Respiratory Distress Syndrome [see Warnings and Precautions (5.4)]
- •
- Gadolinium Retention [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The adverse reactions described in this section reflect Gadavist exposure in 7,713 subjects (including 184 pediatric patients, ages 0 to 17 years) with the majority receiving the recommended dose. Approximately 52% of the subjects were male and the ethnic distribution was 62% Caucasian, 28% Asian, 5% Hispanic, 2.5% Black, and 2.5% patients of other ethnic groups. The average age was 56 years (range from 1 week to 93 years).
Overall, approximately 4% of subjects reported one or more adverse reactions during a follow-up period that ranged from 24 hours to 7 days after Gadavist administration.
Adverse reactions associated with the use of Gadavist were usually mild to moderate in severity and transient in nature.
Table 2 lists adverse reactions that occurred in ≥ 0.1% subjects who received Gadavist.
Table 2: Adverse Reactions Reaction
Rate (%)
n=7713
Headache
1.7
Nausea
1.2
Dizziness
0.5
Dysgeusia
0.4
Feeling Hot
0.4
Injection site reactions
0.4
Vomiting
0.4
Rash (includes generalized, macular, papular, pruritic)
0.3
Erythema
0.2
Paresthesia
0.2
Pruritus (includes generalized)
0.2
Dyspnea
0.1
Urticaria
0.1
Adverse reactions that occurred with a frequency of < 0.1% in subjects who received Gadavist include: hypersensitivity/anaphylactic reaction, loss of consciousness, convulsion, parosmia, tachycardia, palpitation, dry mouth, malaise and feeling cold.
Close6.2 Postmarketing Experience
The following additional adverse reactions have been identified during postmarketing use of Gadavist or other GBCAs. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- •
- Cardiac arrest
- •
- Nephrogenic Systemic Fibrosis (NSF)
- •
- Hypersensitivity reactions (anaphylactic shock, circulatory collapse, respiratory arrest, bronchospasm, cyanosis, oropharyngeal swelling, laryngeal edema, blood pressure increased, chest pain, angioedema, conjunctivitis, hyperhidrosis, cough, sneezing, burning sensation, and pallor)
- •
- Respiratory, Thoracic, and Mediastinal Disorders: Acute respiratory distress syndrome, pulmonary edema
- •
- General Disorders and Administration Site Conditions: Adverse reactions with variable onset and duration have been reported after GBCA administration. These include fatigue, asthenia, pain syndromes, and heterogeneous clusters of symptoms in the neurological, cutaneous, and musculoskeletal systems.
- •
- Skin: Gadolinium associated plaques
- •
- Gastrointestinal Disorders: Acute pancreatitis with onset within 48 hours after GBCA administration
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - GBCAs cross the placenta and result in fetal exposure and gadolinium retention. The human data on the association between GBCAs and adverse fetal outcomes are ...
8.1 Pregnancy
Risk Summary
GBCAs cross the placenta and result in fetal exposure and gadolinium retention. The human data on the association between GBCAs and adverse fetal outcomes are limited and inconclusive (see Data). In animal reproduction studies, although teratogenicity was not observed, embryolethality was observed in monkeys, rabbits and rats receiving intravenous gadobutrol during organogenesis at doses 8 times and above the recommended human dose. Retardation of embryonal development was observed in rabbits and rats receiving intravenous gadobutrol during organogenesis at doses 8 and 12 times, respectively, the recommended human dose (see Data). Because of the potential risks of gadolinium to the fetus, use Gadavist only if imaging is essential during pregnancy and cannot be delayed.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and is 15 to 20%, respectively.
Data
Human Data.
Contrast enhancement is visualized in the placenta and fetal tissues after maternal GBCA administration.
Cohort studies and case reports on exposure to GBCAs during pregnancy have not reported a clear association between GBCAs and adverse effects in the exposed neonates. However, a retrospective cohort study, comparing pregnant women who had a GBCA MRI to pregnant women who did not have an MRI, reported a higher occurrence of stillbirths and neonatal deaths in the group receiving GBCA MRI. Limitations of this study include a lack of comparison with non-contrast MRI and lack of information about the maternal indication for MRI. Overall, these data preclude a reliable evaluation of the potential risk of adverse fetal outcomes with the use of GBCAs in pregnancy.
Animal Data
Gadolinium Retention
GBCAs administered to pregnant non-human primates (0.1 mmol/kg on gestational days 85 and 135) result in measurable gadolinium concentration in the offspring in bone, brain, skin, liver, kidney, and spleen for at least 7 months. GBCAs administered to pregnant mice (2 mmol/kg daily on gestational days 16 through 19) result in measurable gadolinium concentrations in the pups in bone, brain, kidney, liver, blood, muscle, and spleen at one month postnatal age.
Reproductive Toxicology
Embryolethality was observed when gadobutrol was administered intravenously to monkeys during organogenesis at doses 8 times the recommended single human dose (based on body surface area); gadobutrol was not maternally toxic or teratogenic at this dose. Embryolethality and retardation of embryonal development also occurred in pregnant rats receiving maternally toxic doses of gadobutrol (≥ 7.5 mmol/kg body weight; equivalent to 12 times the human dose based on body surface area) and in pregnant rabbits (≥ 2.5 mmol/kg body weight; equivalent to 8 times the recommended human dose based on body surface area). In rabbits, this finding occurred without evidence of pronounced maternal toxicity and with minimal placental transfer (0.01% of the administered dose detected in the fetuses).
Because pregnant animals received repeated daily doses of Gadavist, their overall exposure was significantly higher than that achieved with the standard single dose administered to humans
8.2 Lactation
Risk Summary
There are no data on the presence of gadobutrol in human milk, the effects on the breastfed infant, or the effects on milk production. However, published lactation data on other GBCAs indicate that 0.01 to 0.04% of the maternal gadolinium dose is present in breast milk and there is limited GBCA gastrointestinal absorption in the breast-fed infant. Gadobutrol is present in rat milk (see Data). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Gadavist and any potential adverse effects on the breastfed infant from Gadavist or from the underlying maternal condition.
Data
In lactating rats receiving 0.5 mmol/kg of intravenous [153Gd]-gadobutrol, 0.01% of the total administered radioactivity was transferred to the pup via maternal milk within 3 hours after administration, and the gastrointestinal absorption is poor (approximately 5% of the dose orally administered was excreted in the urine).
8.4 Pediatric Use
The safety and effectiveness of Gadavist have been established in pediatric patients, including term neonates, for use with MRI to detect and visualize areas with disrupted blood brain barrier and/or abnormal vascularity of the central nervous system and for use in MRA to evaluate known or suspected supra-aortic or renal artery disease. Use of Gadavist in these indications is supported by adequate and well-controlled studies in adults and supportive imaging data in two studies in 135 patients 2 to less than 18 years of age and 44 patients less than 2 years of age with CNS and non-CNS lesions, and pharmacokinetic data in 130 patients 2 to less than 18 years of age and 43 patients less than 2 years of age, including term neonates [see Clinical Pharmacology (12.3) and Clinical Studies (14.1)]. The frequency, type, and severity of adverse reactions in pediatric patients were similar to adverse reactions in adults [see Adverse Reactions (6.1)]. No dose adjustment according to age is necessary in pediatric patients [see Dosage and Administration (2.1), Clinical Pharmacology (12.3), and Clinical Studies (14.1)]. The safety and effectiveness of Gadavist have not been established in preterm neonates for any indication or in pediatric patients of any age for use with MRI to assess the presence and extent of malignant breast disease, or for use in CMRI to assess myocardial perfusion (stress, rest) and late gadolinium enhancement in patients with known or suspected coronary artery disease (CAD).
NSF Risk
No case of NSF associated with Gadavist or any other GBCA has been identified in pediatric patients ages 6 years and younger. Pharmacokinetic studies suggest that clearance of Gadavist is similar in pediatric patients and adults, including pediatric patients age younger than 2 years. No increased risk factor for NSF has been identified in juvenile animal studies of gadobutrol. Normal estimated GFR (eGFR) is around 30 mL/min/1.73m2 at birth and increases to mature levels around 1 year of age, reflecting growth in both glomerular function and relative body surface area. Clinical studies in pediatric patients younger than 1 year of age have been conducted in patients with the following minimum eGFR: 31 mL/min/1.73m2 (age 2 to 7 days), 38 mL/min/1.73m2 (age 8 to 28 days), 62 mL/min/1.73m2 (age 1 to 6 months), and 83 mL/min/1.73m2 (age 6 to 12 months).
8.5 Geriatric Use
In clinical studies of Gadavist, 1,377 patients were 65 years of age and over, while 104 patients were 80 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, use of Gadavist in elderly patients should be cautious, reflecting the greater frequency of impaired renal function and concomitant disease or other drug therapy. No dose adjustment according to age is necessary in this population.
Close8.6 Renal Impairment
Prior to administration of Gadavist, screen all patients for renal dysfunction by obtaining a history and/or laboratory tests [see Warnings and Precautions (5.2)]. No dosage adjustment is recommended for patients with renal impairment.
Gadavist can be removed from the body by hemodialysis [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGEThe maximum dose of Gadavist tested in healthy volunteers, 1.5 mL/kg body weight (1.5 mmol/kg; 15 times the recommended dose), was tolerated in a manner similar to lower doses. Gadavist can be ...
-
11 DESCRIPTIONGadavist (gadobutrol) injection is a paramagnetic macrocyclic contrast agent administered for magnetic resonance imaging. The chemical name for gadobutrol is ...
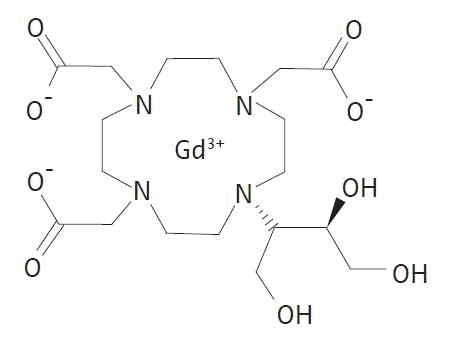
Gadavist (gadobutrol) injection is a paramagnetic macrocyclic contrast agent administered for magnetic resonance imaging. The chemical name for gadobutrol is 10–[(1SR,2RS)–2,3–dihydroxy–1–hydroxymethylpropyl]–1,4,7,10–tetraazacyclododecane–1,4,7–triacetic acid, gadolinium complex. Gadobutrol has a molecular formula of C18H31GdN4O9 and a molecular weight of 604.72.
Gadavist is a sterile, clear, colorless to pale yellow solution containing 604.72 mg (1.0 mmol) of gadobutrol per mL as the active ingredient with 0.513 mg of calcobutrol sodium, 1.211 mg of trometamol, hydrochloric acid (for pH adjustment) and water for injection. Gadavist contains no preservatives.
The main physicochemical properties of Gadavist (1 mmol/mL solution for injection) are listed below:
Density (g/mL at 37°C)
1.3
Osmolarity at 37°C (mOsm/L solution)
1117
Osmolality at 37°C (mOsm/kg H2O)
1603
Viscosity at 37°C (mPa·s)
4.96
pH
6.6–8
The thermodynamic stability constants for gadobutrol (log Ktherm and log Kcond at pH 7.4) are 21.8 and 15.3, respectively.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - In MRI, visualization of normal and pathological tissue depends in part on variations in the radiofrequency signal intensity that occurs with: • Differences in proton ...
12.1 Mechanism of Action
In MRI, visualization of normal and pathological tissue depends in part on variations in the radiofrequency signal intensity that occurs with:
- •
- Differences in proton density
- •
- Differences of the spin-lattice or longitudinal relaxation times (T1)
- •
- Differences in the spin-spin or transverse relaxation time (T2)
When placed in a magnetic field, Gadavist shortens the T1 and T2 relaxation times. The extent of decrease of T1 and T2 relaxation times, and therefore the amount of signal enhancement obtained from Gadavist, is based upon several factors including the concentration of Gadavist in the tissue, the field strength of the MRI system, and the relative ratio of the longitudinal and transverse relaxation times. At the recommended dose, the T1 shortening effect is observed with greatest sensitivity in T1-weighted magnetic resonance sequences. In T2*-weighted sequences the induction of local magnetic field inhomogeneities by the large magnetic moment of gadolinium and at high concentrations (during bolus injection) leads to a signal decrease.
12.2 Pharmacodynamics
Gadavist leads to distinct shortening of the relaxation times even in low concentrations. At pH 7, 37°C and 1.5 T, the relaxivity (r1) - determined from the influence on the relaxation times (T1) of protons in plasma - is 5.2 L/(mmol·sec) and the relaxivity (r2) - determined from the influence on the relaxation times (T2) - is 6.1 L/(mmol·sec). These relaxivities display only slight dependence on the strength of the magnetic field. The T1 shortening effect of paramagnetic contrast agents is dependent on concentration and r1 relaxivity (see Table 3). This may improve tissue visualization.
Table 3: Relaxivity (r1) of Gadolinium Chelates at 1.5 T Gadolinium-Chelate
r1 (L·mmol -1 ·s-1)
Gadobenate
6.3
Gadobutrol
5.2
Gadodiamide
4.3
Gadofosveset
16
Gadopentetate
4.1
Gadoterate
3.6
Gadoteridol
4.1
Gadoversetamide
4.7
Gadoxetate
6.9
r1 relaxivity in plasma at 37°C
Compared to 0.5 molar gadolinium-based contrast agents, the higher concentration of Gadavist results in half the volume of administration and a more compact contrast bolus injection. At the site of imaging, the relative height and width of the time intensity curve for Gadavist varies as a function of imaging location and multiple patient, injection, and device-specific factors.
Gadavist is a water-soluble, hydrophilic compound with a partition coefficient between n-butanol and buffer at pH 7.6 of about 0.006.
Close12.3 Pharmacokinetics
Distribution
After intravenous administration, gadobutrol is rapidly distributed in the extracellular space. After a gadobutrol dose of 0.1 mmol/kg body weight, an average level of 0.59 mmol gadobutrol/L was measured in plasma 2 minutes after the injection and 0.3 mmol gadobutrol/L 60 minutes after the injection. Gadobutrol does not display any particular protein binding. Following GBCA administration, gadolinium is present for months or years in brain, bone, skin, and other organs [see Warnings and Precautions (5.5)].
Elimination
Values for AUC, body weight normalized plasma clearance and half-life are given in Table 4, below.
Gadobutrol is excreted in an unchanged form via the kidneys. In healthy subjects, renal clearance of gadobutrol is 1.1 to 1.7 mL/(min∙kg) and thus comparable to the renal clearance of inulin, confirming that gadobutrol is eliminated by glomerular filtration.
Within two hours after intravenous administration more than 50% and within 12 hours more than 90% of the given dose is eliminated via the urine. Extra-renal elimination is negligible.
Specific Populations
Geriatric
A single IV dose of 0.1 mmol/kg Gadavist was administered to 15 elderly and 16 non-elderly subjects. AUC was slightly higher and clearance slightly lower in elderly subjects as compared to non-elderly subjects [see Use in Specific Populations (8.5)].
Pediatric
The pharmacokinetics of gadobutrol were evaluated in two studies in a total of 130 patients age 2 to less than 18 years and in 43 patients less than 2 years of age (including term neonates). Patients received a single intravenous dose of 0.1 mmol/kg of Gadavist. The pharmacokinetic profile of gadobutrol in pediatric patients is similar to that in adults, resulting in similar values for AUC, body weight normalized plasma clearance, as well as elimination half-life. Approximately 99% (median value) of the dose was recovered in urine within 6 hours (this information was derived from the 2 to less than 18 year old age group).
Table 4: Pharmacokinetics by Age Group (Median [Range]) 0 to < 2 years
2 to 6 years
7 to 11 years
12 to < 18 years
Adults
N=43
N=45
N=39
N=46
N=93
AUC (µmolxh/L)
781
[513, 1891]
846
[412, 1331]
1025
[623, 2285]
1237
[946, 2211]
1072
[667, 1992]
CL (L/h/kg)
0.128
[0.053, 0.195]
0.119
[0.080, 0.215]
0.099
[0.043, 0.165]
0.081
[0.046, 0.103]
0.094
[0.051, 0.150]
t1/2 (h)
2.91
[1.60, 12.4]
1.91
[1.04, 2.70]
1.66
[0.91, 2.71]
1.68
[1.31, 2.48]
1.80
[1.20, 6.55]
C20 (µmol/L)
367
[280, 427]
421
[369, 673]
462
[392, 760]
511
[387, 1077]
441
[281, 829]
Renal Impairment
In patients with impaired renal function, the serum half-life of gadobutrol is prolonged and correlated with the reduction in creatinine clearance.
After intravenous injection of 0.1 mmol gadobutrol/kg body weight, the elimination half-life was 5.8 ± 2.4 hours in mild to moderately impaired patients (80 > CLCR > 30 mL/min) and 17.6 ± 6.2 hours in severely impaired patients not on dialysis (CLCR < 30 mL/min). The mean AUC of gadobutrol in patients with normal renal function was 1.1 ± 0.1 mmol∙h/L, compared to 4.0 ± 1.8 mmol∙h/L in patients with mild to moderate renal impairment and 11.5 ± 4.3 mmol∙h/L in patients with severe renal impairment.
Complete recovery in the urine was seen in patients with mild or moderate renal impairment within 72 hours. In patients with severely impaired renal function about 80% of the administered dose was recovered in the urine within 5 days.
For patients receiving hemodialysis, physicians may consider the prompt initiation of hemodialysis following the administration of Gadavist in order to enhance the contrast agent’s elimination. Sixty-eight percent (68%) of gadobutrol is removed from the body after the first dialysis, 94% after the second dialysis, and 98% after the third dialysis session. [see Warnings and Precautions (5.2) and Use in Specific Populations (8.6).]
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity studies of gadobutrol have been conducted. Gadobutrol was not mutagenic in in vitro reverse mutation tests in ...
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity studies of gadobutrol have been conducted.
Gadobutrol was not mutagenic in in vitro reverse mutation tests in bacteria, in the HGPRT (hypoxanthine-guanine phosphoribosyl transferase) test using cultured Chinese hamster V79 cells, or in chromosome aberration tests in human peripheral blood lymphocytes, and was negative in an in vivo micronucleus test in mice after intravenous injection of 0.5 mmol/kg.
Gadobutrol had no effect on fertility and general reproductive performance of male and female rats when given in doses 12.2 times the human equivalent dose (based on body surface area).
Close13.2 Animal Toxicology and/or Pharmacology
Local intolerance reactions, including moderate irritation associated with infiltration of inflammatory cells was observed after paravenous administration to rabbits, suggesting the possibility of occurrence of local irritation if the contrast medium leaks around veins in a clinical setting [see Warnings and Precautions (5.7)].
-
14 CLINICAL STUDIES14.1 MRI of the CNS - Patients referred for MRI of the central nervous system with contrast were enrolled in two clinical trials that evaluated the visualization characteristics of lesions. In ...
14.1 MRI of the CNS
Patients referred for MRI of the central nervous system with contrast were enrolled in two clinical trials that evaluated the visualization characteristics of lesions. In both studies, patients underwent a baseline, pre-contrast MRI prior to administration of Gadavist at a dose of 0.1 mmol/kg, followed by a post-contrast MRI. In Study A, patients also underwent an MRI before and after the administration of gadoteridol. The studies were designed to demonstrate superiority of Gadavist MRI to non-contrast MRI for lesion visualization. For both studies, pre-contrast and pre-plus-post contrast images (paired images) were independently evaluated by three readers for contrast enhancement and border delineation using a scale of 1 to 4, and for internal morphology using a scale of 1 to 3 (Table 5). Lesion counting was also performed to demonstrate non-inferiority of paired Gadavist image sets to pre-contrast MRI. Readers were blinded to clinical information.
Table 5: Primary Endpoint Visualization Scoring System Score
Visualization Characteristics
Contrast Enhancement
Border Delineation
Internal Morphology
1
None
None
Poorly visible
2
Weak
Moderate
Moderately visible
3
Clear
Clear but incomplete
Sufficiently visible
4
Clear and bright
Clear and complete
N/A
Efficacy was determined in 657 subjects. The average age was 49 years (range 18 to 85 years) and 42% were male. The ethnic representations were 39% Caucasian, 4% Black, 16% Hispanic, 38% Asian, and 3% of other ethnic groups.
Table 6 shows a comparison of visualization results between paired images and pre-contrast images. Gadavist provided a statistically significant improvement for each of the three lesion visualization parameters when averaged across three independent readers for each study.
Table 6: Visualization Endpoint Results of Central Nervous System Adult MRI Studies with 0.1 mmol/kg Gadavist Endpoint
Study A
N=336Study B
N=321Pre-contrast
Paired
Difference*
Pre-contrast
Paired
Difference
Contrast Enhancement
0.97
2.26
1.29†
0.93
2.86
1.94†
Border Delineation
1.98
2.58
0.60†
1.92
2.94
1.02†
Internal Morphology
1.32
1.93
0.60†
1.57
2.35
0.78†
Average # Lesions Detected
8.08
8.25
0.17‡
2.65
2.97
0.32§
Performances of Gadavist and gadoteridol for visualization parameters were similar. Regarding the number of lesions detected, Study B met the prespecified noninferiority margin of -0.35 for paired read versus pre-contrast read while in Study A, Gadavist and gadoteridol did not.
For the visualization endpoints contrast enhancement, border delineation, and internal morphology, the percentage of patients scoring higher for paired images compared to pre-contrast images ranged from 93% to 99% for Study A, and 95% to 97% for Study B. For both studies, the mean number of lesions detected on paired images exceeded that of the pre-contrast images; 37% for Study A and 24% for Study B. There were 29% and 11% of subjects in which the pre-contrast images detected more lesions for Study A and Study B, respectively.
The percentage of patients whose average reader mean score changed by ≤ 0, up to 1, up to 2, and ≥ 2 scoring categories presented in Table 5 is shown in Table 7. The categorical improvement of (≤ 0) represents higher (< 0) or identical (= 0) scores for the pre-contrast read, the categories with scores > 0 represent the magnitude of improvement seen for the paired read.
Table 7: Primary Endpoint Visualization Categorical Improvement for Average Reader Study A
N=336Study B
N=321Endpoint
Categorical Improvement
(Paired – Pre-Contrast) %Categorical Improvement
(Paired – Pre-Contrast) %≤ 0
> 0 – < 1
1 – < 2
≥ 2
≤ 0
- > 0 – < 1
1 – < 2
≥ 2
Contrast Enhancement
1
30
55
13
3
6
34
57
Border Delineation
7
73
18
1
5
38
51
5
Internal Morphology
4
79
17
0
5
61
33
1
For both studies, the improvement of visualization endpoints in paired Gadavist images compared to pre-contrast images resulted in improved assessment of normal and abnormal CNS anatomy.
Pediatric Patients
Two studies in 44 pediatric patients age younger than 2 years and 135 pediatric patients age 2 to less than 18 years with CNS and non-CNS lesions supported extrapolation of adult CNS efficacy findings. For example, comparing pre vs paired pre- and post-contrast images, investigators selected the best of four descriptors under the heading, “Visualization of lesion-internal morphology (lesion characterization) or homogeneity of vessel enhancement” for 27/44 (62% = pre) vs 43/44 (98% = paired) MR images from patients age 0 to less than 2 years and 106/135 (78% = pre) vs 108/135 (80% = paired) MR images from patients age 2 to less than 18 years.
14.2 MRI of the Breast
Patients with recently diagnosed breast cancer were enrolled in two identical clinical trials to evaluate the ability of Gadavist to assess the presence and extent of malignant breast disease prior to surgery. Patients underwent non-contrast breast MRI (BMR) prior to Gadavist (0.1 mmol/kg) breast MRI. BMR images and Gadavist BMR (combined contrast plus non-contrast) images were independently evaluated in each study by three readers blinded to clinical information. In separate reading sessions the BMR images and Gadavist BMR images were also interpreted together with X-ray mammography images (XRM).
The studies evaluated 787 patients: Study 1 enrolled 390 women with an average age of 56 years, 74% were white, 25% Asian, 0.5% black, and 0.5% other; Study 2 enrolled 396 women and 1 man with an average age of 57 years, 71% were white, 24% Asian, 3% black, and 2% other.
The readers assessed 5 regions per breast for the presence of malignancy using each reading modality. The readings were compared to an independent standard of truth (SoT) consisting of histopathology for all regions where excisions were made and tissue evaluated. XRM plus ultrasound was used for all other regions.
The assessment of malignant disease was performed using a region based within-subject sensitivity. Sensitivity for each reading modality was defined as the mean of the percentage of malignant breast regions correctly interpreted for each subject. The within-subject sensitivity of Gadavist BMR was superior to that of BMR. The lower bound of the 95% Confidence Interval (CI) for the difference in within-subject sensitivity ranged from 19% to 42% for Study 1 and from 12% to 27% for Study 2. The within-subject sensitivity for Gadavist BMR and BMR as well as for Gadavist BMR plus XRM and BMR plus XRM is presented in Table 8.
Table 8: Sensitivity of Gadavist BMR for Detection of Malignant Breast Disease Study 1
Study 2
Sensitivity (%)
N=388 Patients
Sensitivity (%)
N=390 Patients
Reader
BMR
BMR + XRM
Gadavist BMR
Gadavist BMR
+XRM
Reader
BMR
BMR
+ XRMGadavist BMR
Gadavist BMR
+XRM1
37
71
83
84
4
73
83
87
90
2
49
76
80
83
5
57
81
89
90
3
63
75
87
87
6
55
80
86
88
Specificity was defined as the percentage of non-malignant breasts correctly identified as non-malignant. The lower limit of the 95% confidence interval for specificity of Gadavist BMR was greater than 80% for 5 of 6 readers. (Table 9)
Table 9: Specificity of Gadavist BMR in Non-Malignant Breasts Study 1
Study 2
Specificity (%)
N=372 Patients
Specificity (%)
N=367 Patients
Reader
Gadavist BMR
Lower Limit
95% CI
Reader
Gadavist BMR
Lower Limit
95% CI
1
86
82
4
92
89
2
95
93
5
84
80
3
89
85
6
83
79
Three additional readers in each study read XRM alone. For these readers over both studies, sensitivity ranged from 68% to 73% and specificity in non-malignant breasts ranged from 86% to 94%.
In breasts with malignancy, a false positive detection rate was calculated as the percentage of subjects for which the readers assessed a region as malignant which could not be verified by SoT. The false positive detection rates for Gadavist BMR ranged from 39% to 53% (95% CI Upper Bounds ranged from 44% to 58%).
14.3 MRA
Patients with known or suspected disease of the supra-aortic arteries (for evaluation up to but excluding the basilar artery) were enrolled in Study C, and patients with known or suspected disease of the renal arteries were enrolled in Study D. In both studies, non-contrast, 2D time-of-flight (ToF) magnetic resonance angiography (MRA) was performed prior to Gadavist MRA using a single intravenous injection of 0.1 mmol/kg. The injection rate of 1.5 mL/second was selected to extend the injection duration to at least half of the imaging duration. Imaging was performed with parallel-channel, 1.5T MRI devices and an automatic bolus tracking technique to trigger the image acquisition following Gadavist administration using elliptically encoded, T1-weighted, 3D gradient-echo image acquisition and single breath hold. Three central readers blinded to clinical information interpreted the ToF and Gadavist MRA images. Three additional central readers interpreted separately acquired computed tomographic angiography (CTA) images, which were used as the standard of reference (SoR) in each study.
The studies included 749 subjects: 457 were evaluated in Study C, with an average age of 68 (range 25–93); 64% were male; 80% white, 28% black, and 16% Asian. An additional 292 subjects were evaluated in Study D, with an average age of 55 (range 18–88); 54% were male; 68% white, 7% black, and 22% Asian.
Efficacy was evaluated based on anatomical visualization and performance for distinguishing between normal and abnormal anatomy. The visualization metric depended on whether readers selected, “Yes, it can be visualized along its entire length...” when responding to the question, “Is this segment assessable?” Twenty-one segments in Study C and six segments in Study D were presented per subject to each reader. The performance metrics, sensitivity and specificity, depended on digital caliper-based quantitation of arterial narrowing in visualized, non-occluded, abnormal-appearing segments. Significant stenosis was defined as at least 70% in Study C and 50% in Study D. Performance of Gadavist MRA compared to ToF MRA was calculated using an imputation method for non-visualized segments by assigning them as a 50% match with SoR and a 50% mismatch. Performance of Gadavist MRA compared to a pre-specified threshold of 50% was calculated after excluding non-visualized segments. Measurement variability and visualization of accessory renal arteries was also evaluated.
Results were analyzed for each of the three central readers.
Table 10: Visualization, Sensitivity, Specificity - *
- Number of segments varied between readers; number for majority-reader shown.
- †
- Standard of Reference based on aggregate interpretation of three central CTA readers.
- ‡
- 95.1/95% (Study C/D) confidence interval for two-sided comparison.
- §
- 90.1/90% (Study C/D) confidence interval for one-sided comparison against non-inferiority margin of -7.5.
STUDY C: SUPRA-AORTIC ARTERIES (457 patients)
Performance at the segment level
9597*segments of which 158* were positive for stenosis by SoR†
VISUALIZATION (%)
SENSITIVITY (%)
SPECIFICITY (%)
READER
GAD
MRA
ToF MRA
GAD - ToF
(CI‡)
GAD MRA
ToF MRA
GAD - ToF (CI§)
GAD MRA
ToF MRA
GAD - ToF (CI§)
1
88
24
64
(61, 67)
60
54
6
(-4, 14)
92
62
30
(29, 32)
2
95
75
20
(18, 21)
60
54
6
(-3, 14)
95
85
10
(9, 11)3
97
82
15
(13, 17)
58
55
3
(-4, 11)
97
89
8
(7, 9)
STUDY D: RENAL ARTERIES (292 patients)
Performance at the segment level
17521 segments of which 1331 were positive for stenosis by SoR2
4
98
82
16
(13, 20)
52
51
1(-9, 11)
94
83
11
(9, 14)
5
96
72
24
(21, 28)
54
39
15
(6, 24)
95
85
10
(8, 12)6
96
78
17
(14, 21)
53
50
3
(-6, 12)
94
81
13
(11, 16)
GAD MRA = Post-contrast Gadavist Magnetic Resonance Angiography, ToF = Non-contrast 2D-Time of Flight.
For all three supra-aortic artery readers in Study C, the lower bound of confidence for the sensitivity of Gadavist MRA did not exceed 54%. For all three renal artery readers in Study D, the lower bound of confidence for the sensitivity of Gadavist MRA did not exceed 46%.
Measurement Variability
For both MRA and CTA, readers varied in the quantity of narrowing they assigned to the same arterial segments. Table 11 shows the percentage of patients in whom the measurement range was 30% or greater for the left or right internal carotid and proximal renal artery segments. There were approximately four measurements per patient segment, one from the site and three central readers. Measurement variability was high for both CTA and MRA, but numerically lower for Gadavist compared to non-contrast ToF MRA.
Table 11: Percent of Patients with Range ≥ 30%, ≥ 50%, ≥ 70% for Measurement of Stenoses and Normal Vessel Diameters Internal Carotid
Proximal Main Renal
N
≥ 30% Carotid
≥ 50%
≥ 70%
N
≥ 30%
≥ 50%
≥ 70%
CTA
456
40
11
4
292
59
33
9
ToF MRA
443
55
22
9
270
44
22
9
Gadavist MRA
454
47
13
4
286
34
14
4
Visualization of Accessory Renal Arteries for Surgical Planning and Renal Donor Evaluation (Study D only)
Of 1752 main arteries visualized by the central CTA readers, 266 (15%) were also associated with positive visualization of at least one accessory (duplicate) artery. With the central MRA readers, the comparable rates were 232 of 1752 (13%) for Gadavist MRA compared to 53 of 1752 (3%) for ToF MRA.
Close14.4 Cardiac MRI
Two studies similar in design, Study E and Study F, evaluated the sensitivity and specificity of Gadavist cardiac MRI (CMRI) for detection of coronary artery disease (CAD) in adult patients with known or suspected CAD. Patients were excluded from study if they had a history of coronary artery bypass grafting, or if it was known in advance that they were unable to hold their breath, or had atrial fibrillation or other arrhythmia likely to prevent electrocardiogram-gated CMRI. The studies were multi-center, open-label, and evaluated 764 subjects for efficacy: 376 in Study E, with an average age of 59 (range 20–84); 69% male; 74% white, 1% black, and 25% Asian; and 388 subjects in Study F, with an average age of 59 (range 23–82); 61% male; 67% white, 17% black, and 12% Asian.
All subjects underwent dynamic first-pass Gadavist imaging during vasodilator stress, followed ~10 minutes later by dynamic first-pass Gadavist imaging at rest, followed ~5 minutes later with imaging during a period of gradual Gadavist washout from the myocardium (late gadolinium enhancement, LGE). Imaging was performed on 1.5 T or 3.0 T MRI devices equipped with multichannel surface coils to support accelerated acquisitions with parallel imaging, T1-weighted, 2D gradient-echo, dynamic acquisition of perfusion with at least 3 slices per heartbeat. Gadavist was administered intravenously at a rate of ~4 mL/second as two separate bolus injections (0.05 mmol/kg each), the first at peak pharmacologic stress (~3 minutes after start of ongoing adenosine infusion, or immediately after completion of regadenoson administration, at approved doses). No additional Gadavist was administered for LGE imaging.
Images were read by three independent readers blinded to clinical information. Reader detection of CAD depended on visually detecting defective perfusion or scar on Gadavist CMRI (stress, rest, LGE) imaging. Quantitative coronary angiography (QCA) was used to measure intraluminal narrowing and served as the standard of reference (SoR). Computed tomographic angiography (CTA) was used as the SoR if disease could be unequivocally excluded, and no coronary angiography (CA) was available. The left ventricular myocardium was divided into six regions. Readers provided per-region (CMRI, CTA) and per-artery (QCA) interpretations for each subject. Subject-level endpoints reflected each subject’s most abnormal localized finding.
The sensitivity results for Gadavist CMRI to detect CAD defined as either maximum stenosis ≥ 50% or ≥ 70% by QCA are presented in Table 12. For each reader, sensitivity of Gadavist CMRI larger than 60% can be concluded if the lower 95% confidence limit of the sensitivity estimate exceeds the pre-specified threshold of 60%.
Table 12: Sensitivity (%) of Gadavist-CMRI for Detection of CAD in Patients with Maximum Stenosis* of ≥ 50% and ≥ 70%
Study E
Study F
≥ 50%
N=141
≥ 70%
N=108
≥ 50%
N=150
≥ 70%
N=105
Reader 1**
77 (69, 83)***
90 (83, 95)
65 (57, 72)
77 (68, 85)
Reader 2**
65 (57, 73)
80 (71, 87)
56 (48, 64)
71 (62, 80)
Reader 3**
65 (56, 72)
79 (70, 86)
61 (53, 69)
76 (67, 84)
* Stenosis determined by Quantitative Coronary Angiography (QCA)
** CMRI images were assessed by six independent blinded readers, three in each study.
*** The bolded value represents the lower limit of the 95% confidence interval, which is compared to a pre-specified threshold of 60% for evaluation of sensitivity.
The specificity results for Gadavist CMRI to detect CAD defined as either maximum stenosis ≥ 50% or ≥ 70% by QCA are presented in Table 13. For each reader, specificity of Gadavist CMRI larger than 55% can be concluded if the lower 95% confidence limit of the specificity estimate exceeds the pre-specified threshold of 55%.
Table 13: Specificity (%) of Gadavist CMRI for Exclusion of CAD in Patients with Maximum Stenosis* of ≥ 50% and ≥ 70%
Study E
Study F
≥ 50%
N=235
≥ 70%
N=268
≥ 50%
N=239
≥ 70%
N=283
Reader 1**
85 (80, 89)***
83 (78, 87)
85 (80, 90)
82 (77, 86)
Reader 2**
92 (88, 95)
91 (87, 94)
89 (84, 92)
87 (83, 91)
Reader 3**
92 (88, 95)
91 (87, 94)
90 (85, 93)
87 (82, 91)
* Stenosis determined by Quantitative Coronary Angiography (QCA)
** CMRI images were assessed by six independent blinded readers, three in each study.
*** The bolded value represents the lower limit of the 95% confidence interval, which is compared to a pre-specified threshold of 55% for evaluation of specificity.
In Study E, among the 33 patients with maximum stenosis by QCA between 50% and <70%, the proportion of Gadavist-CMRI positive detections of CAD ranged from 15% to 33% . In Study F, among the 45 patients with maximum stenosis by QCA between 50% and < 70%, the proportion of Gadavist-CMRI positive detections of CAD ranged from 20% to 35%. The results of Gadavist-CMRI reads to detect CAD in patients with maximum stenosis between 50% and < 70% are summarized in Table 14.
Table 14: Gadavist-CMRI Detection of CAD in Patients with Maximum Stenosis* between 50% and < 70%
Study E (n=33)
Study F (n=45)
Gadavist-CMRI positive
Gadavist-CMRI positive
Reader 1**
11 (33%)
16 (35%)
Reader 2**
5(15%)
9 (20%)
Reader 3**
6(18%)
12 (26%)
* Stenosis determined by Quantitative Coronary Angiography (QCA).
**CMRI images were assessed by six independent blinded readers, three in each study.
Left Mainstem Stenosis (LMS)
The studies did not include sufficient numbers of subjects to characterize the performance of Gadavist CMRI for detection of LMS, a subgroup at high risk from false negative reads. In Studies E and F, only three subjects had isolated LMS stenosis >50%. In two of the three cases, the CMRI was interpreted as normal by at least two of the three readers (false negative). Sixteen subjects had LMS stenosis >50% (including subjects with isolated LMS stenosis and subjects with LMS stenosis in addition to stenoses elsewhere). . In five of these sixteen cases, the CMR was interpreted as normal by at least two of the three readers (false negative).
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Gadavist is a sterile, clear and colorless to pale yellow solution containing 604.72 mg gadobutrol per mL (equivalent to 1 mmol gadobutrol) per mL. Gadavist is supplied in the ...
16.1 How Supplied
Gadavist is a sterile, clear and colorless to pale yellow solution containing 604.72 mg gadobutrol per mL (equivalent to 1 mmol gadobutrol) per mL. Gadavist is supplied in the following sizes:
Single-Dose Containers (Vials)
- •
- 2 mL single-dose vials, rubber stoppered in cartons of 3, Boxes of 15 (NDC 50419-325-37)
- •
- 7.5 mL single-dose vials, rubber stoppered in cartons of 10, Boxes of 20 (NDC 50419-325-11)
- •
- 10 mL single-dose vials, rubber stoppered, in cartons of 10, Boxes of 20 (NDC 50419-325-12)
- •
- 15 mL single-dose vials, rubber stoppered, in cartons of 10, Boxes of 20 (NDC 50419-325-13)
Close16.2 Storage and Handling
Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F) [see USP Controlled Room Temperature].
Should freezing occur, Gadavist should be brought to room temperature before use. If allowed to stand at room temperature, Gadavist should return to a clear and colorless to pale yellow solution. Visually inspect Gadavist for particulate matter and discoloration prior to administration. Do not use the solution if it is discolored, if particulate matter is present or if the container appears damaged.
-
17 PATIENT COUNSELING INFORMATION• Advise the patient to read the FDA-approved patient labeling (Medication Guide). Nephrogenic Systemic Fibrosis - Instruct patients to inform their physician if they: • Have a history of ...
- •
- Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Nephrogenic Systemic Fibrosis
Instruct patients to inform their physician if they:
- •
- Have a history of kidney disease and/or liver disease, or
- •
- Have recently received a GBCA
GBCAs increase the risk of NSF among patients with impaired elimination of drugs. To counsel patients at risk of NSF:
- •
- Describe the clinical manifestation of NSF
- •
- Describe procedures to screen for the detection of renal impairment
Instruct the patients to contact their physician if they develop signs or symptoms of NSF following Gadavist administration, such as burning, itching, swelling, scaling, hardening and tightening of the skin; red or dark patches on the skin; stiffness in joints with trouble moving, bending or straightening the arms, hands, legs or feet; pain in the hip bones or ribs; or muscle weakness.
Common Adverse Reactions
Inform patients that they may experience:
- •
- Reactions along the venous injection site, such as mild and transient burning or pain or feeling of warmth or coldness at the injection site
- •
- Side effects of headache, nausea, abnormal taste and feeling hot
Acute Respiratory Distress Syndrome
- •
- Advise patients that acute respiratory distress syndrome (ARDS) has occurred with GADAVIST. Inform patients on the symptoms of the observed ARDS cases, and instruct patients to inform their healthcare provider if they experience these symptoms [see Warnings and Precautions (5.4)].
General Precautions
Gadolinium Retention
- •
- Advise patients that gadolinium is retained for months or years in brain, bone, skin, and other organs in patients with normal renal function. The clinical consequences of retention are unknown. Retention depends on multiple factors and is greater following administration of linear GBCAs than following administration of macrocyclic GBCAs [see Warnings and Precautions (5.5)].
Instruct patients receiving Gadavist to inform their physician if they:
- •
- Are pregnant or breastfeeding
- •
- Have a history of allergic reaction to contrast media, bronchial asthma or allergic respiratory disorder
© 2011, Bayer HealthCare Pharmaceuticals Inc.
All rights reserved.
Manufactured for:
Bayer HealthCare Pharmaceuticals Inc.
Whippany, NJ 07981Manufactured in Germany
Close -
MEDICATION GUIDEMEDICATION GUIDE - GADAVIST - (gad-a-vist) (gadobutrol) Injection for intravenous use - What is the most important information I should know about Gadavist? • GBCAs like Gadavist may ...Close
MEDICATION GUIDE
GADAVIST
(gad-a-vist)(gadobutrol)
Injection for intravenous useWhat is the most important information I should know about Gadavist?
- •
- GBCAs like Gadavist may cause serious side effects including death, coma, encephalopathy, and seizures when it is given intrathecally (injection given into the spinal canal). It is not known if Gadavist is safe and effective with intrathecal use. Gadavist is not approved for this use.
- •
- Gadavist contains a metal called gadolinium. Small amounts of gadolinium can stay in your body including the brain, bones, skin and other parts of your body for a long time (several months to years).
- •
- It is not known how gadolinium may affect you, but so far, studies have not found harmful effects in patients with normal kidneys
- •
- Rarely, patients have reported pains, tiredness, and skin, muscle or bone ailments for a long time, but these symptoms have not been directly linked to gadolinium.
- •
- There are different GBCAs that can be used for your MRI exam. The amount of gadolinium that stays in the body is different for different gadolinium medicines. Gadolinium stays in the body more after Omniscan or Optimark than after Eovist, Magnevist, or MultiHance. Gadolinium stays in the body the least after Dotarem, Gadavist, or ProHance.
- •
- People who get many doses of gadolinium medicines, women who are pregnant and young children may be at increased risk from gadolinium staying in the body.
- •
- Some people with kidney problems who get gadolinium medicines can develop a condition with severe thickening of the skin, muscles and other organs in the body (nephrogenic systemic fibrosis). Your healthcare provider should screen you to see how well your kidneys are working before you receive Gadavist.
What is Gadavist?
- •
- Gadavist is a prescription medicine called a gadolinium-based contrast agent (GBCA). Gadavist, like other GBCAs, is injected into your vein and used with a magnetic resonance imaging (MRI) scanner.
- •
- An MRI exam with a GBCA, including Gadavist, helps your doctor to see problems better than an MRI exam without a GBCA.
- •
- Your doctor has reviewed your medical records and has determined that you would benefit from using a GBCA with your MRI exam.
Do not receive Gadavist if you have had a severe allergic reaction to Gadavist.
Before receiving Gadavist, tell your healthcare provider about all your medical conditions, including if you:
- •
- have had any MRI procedures in the past where you received a GBCA. Your healthcare provider may ask you for more information including the dates of these MRI procedures.
- •
- are pregnant or plan to become pregnant. It is not known if Gadavist can harm your unborn baby. Talk to your healthcare provider about the possible risks to an unborn baby if a GBCA such as Gadavist is received during pregnancy.
- •
- have kidney problems, diabetes, or high blood pressure
- •
- have had an allergic reaction to dyes (contrast agents) including GBCAs
What are the possible side effects of Gadavist?
- •
- See “What is the most important information I should know about Gadavist?”
- •
- Allergic reactions. Gadavist can cause allergic reactions that can sometimes be serious. Your healthcare provider will monitor you closely for symptoms of an allergic reaction.
- •
- A serious lung problem called acute respiratory distress syndrome (ARDS). Call your healthcare provider right away if you have shortness of breath with or without a fever, trouble breathing, or a fast rate of breathing after receiving Gadavist.
The most common side effects of Gadavist include: headache, nausea, and dizziness.
These are not all the possible side effects of Gadavist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Gadavist.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your healthcare provider for information about Gadavist that is written for health professionals.
What are the ingredients in Gadavist?
Active ingredient: gadobutrol
Inactive ingredients: calcobutrol sodium, trometamol, hydrochloric acid (for pH adjustment) and water for injection
Manufactured for Bayer HealthCare Pharmaceuticals Inc.
Manufactured in Germany
© 2011, Bayer HealthCare Pharmaceuticals Inc. All rights reserved.
For more information, go to www.gadavist.com or call 1-888-842-2937.
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 3/2025
-
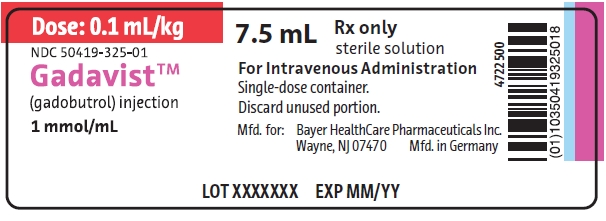
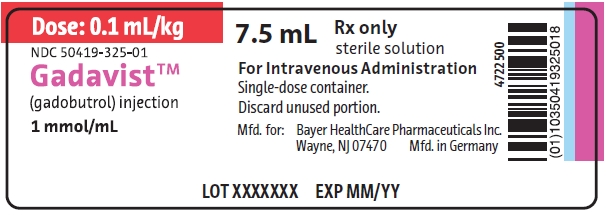
PRINCIPAL DISPLAY PANELGadavist 7.5 mL Label - Dose: 0.1 mL/kg - NDC 50419-325-01 - 7.5 mL - Rx only - sterile solution - Gadavist - (gadobutrol) injection - 1 mmol/mL - For Intravenous Administration. Single-dose ...
Gadavist 7.5 mL Label
Dose: 0.1 mL/kg
NDC 50419-325-01
7.5 mLRx only
sterile solution
Gadavist
(gadobutrol) injection
1 mmol/mLFor Intravenous Administration.
Single-dose container.Discard unused portion.
Close -
INGREDIENTS AND APPEARANCEProduct Information
GADAVIST gadobutrol injection Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50419-325 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GADOBUTROL (UNII: 1BJ477IO2L) (GADOLINIUM CATION (3+) - UNII:AZV954TZ9N) GADOBUTROL 604.72 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROMETHAMINE (UNII: 023C2WHX2V) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50419-325-11 2 in 1 BOX 03/15/2011 1 10 in 1 CARTON 1 7.5 mL in 1 VIAL, SINGLE-DOSE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:50419-325-12 2 in 1 BOX 03/15/2011 2 10 in 1 CARTON 2 10 mL in 1 VIAL, SINGLE-DOSE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:50419-325-13 2 in 1 BOX 03/15/2011 3 10 in 1 CARTON 3 15 mL in 1 VIAL, SINGLE-DOSE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 4 NDC:50419-325-27 5 in 1 BOX 03/15/2011 4 7.5 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 5 NDC:50419-325-28 5 in 1 BOX 03/15/2011 5 10 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 6 NDC:50419-325-29 5 in 1 BOX 03/15/2011 6 15 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 7 NDC:50419-325-72 10 in 1 CARTON 03/15/2011 7 10 mL in 1 VIAL, SINGLE-DOSE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 8 NDC:50419-325-37 15 in 1 BOX 03/15/2011 8 3 in 1 CARTON 8 2 mL in 1 VIAL, SINGLE-DOSE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 9 NDC:50419-325-75 5 in 1 BOX 05/12/2022 9 10 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA201277 03/14/2011 Labeler - Bayer HealthCare Pharmaceuticals Inc. (005436809) Registrant - Bayer HealthCare Pharmaceuticals Inc. (828111489) Establishment Name Address ID/FEI Business Operations Bayer AG 315097875 ANALYSIS(50419-325) , LABEL(50419-325) , MANUFACTURE(50419-325) , PACK(50419-325) Establishment Name Address ID/FEI Business Operations Bayer AG 314398484 API MANUFACTURE(50419-325) , ANALYSIS(50419-325) Establishment Name Address ID/FEI Business Operations Dynamit Nobel GmbH ES 313113144 API MANUFACTURE(50419-325) Establishment Name Address ID/FEI Business Operations Sharp Corporation 143696495 LABEL(50419-325) Establishment Name Address ID/FEI Business Operations Quality Packaging Services International LLC 825078165 LABEL(50419-325)
CloseEstablishment Name Address ID/FEI Business Operations Currenta GmbH & Co. OHG 331575303 ANALYSIS(50419-325)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
GADAVIST- gadobutrol injection
Number of versions: 23
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Mar 28, 2025 | 24 (current) | download |
| Aug 6, 2024 | 23 | download |
| Feb 27, 2024 | 22 | download |
| Feb 14, 2024 | 21 | download |
| Nov 13, 2023 | 20 | download |
| Nov 9, 2022 | 19 | download |
| May 13, 2022 | 18 | download |
| May 6, 2022 | 17 | download |
| Sep 3, 2021 | 16 | download |
| Aug 17, 2020 | 15 | download |
| Jul 17, 2019 | 14 | download |
| Jul 15, 2019 | 13 | download |
| Aug 15, 2018 | 12 | download |
| May 4, 2018 | 11 | download |
| Jul 20, 2017 | 10 | download |
| Apr 29, 2016 | 9 | download |
| Nov 23, 2015 | 8 | download |
| Jan 5, 2015 | 7 | download |
| Jun 13, 2014 | 6 | download |
| Jan 2, 2014 | 5 | download |
| Oct 31, 2013 | 4 | download |
| Aug 7, 2012 | 3 | download |
| Mar 25, 2011 | 2 | download |
RxNorm
GADAVIST- gadobutrol injection
Under Review - Editing is pending for RxNorm. If in scope, these drugs will include RxNorm normal forms when editing is complete.
Get Label RSS Feed for this Drug
GADAVIST- gadobutrol injection
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=3c5101a0-0c7e-4078-8dc3-a57beb9a0e92
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
GADAVIST- gadobutrol injection
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 50419-325-11 |
| 2 | 50419-325-12 |
| 3 | 50419-325-13 |
| 4 | 50419-325-27 |
| 5 | 50419-325-28 |
| 6 | 50419-325-29 |
| 7 | 50419-325-37 |
| 8 | 50419-325-72 |
| 9 | 50419-325-75 |