Label: LMD IN DEXTROSE- dextran 40 injection, solution
LMD IN SODIUM CHLORIDE- dextran 40 injection, solution
- NDC Code(s): 0409-7418-03, 0409-7418-13, 0409-7419-03, 0409-7419-14
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION10% LMD in 0.9% Sodium Chloride Injection - (Dextran 40 in Sodium Chloride Injection, USP) Low Molecular Weight Dextran for Intravenous Administration - Flexible Plastic Container
-
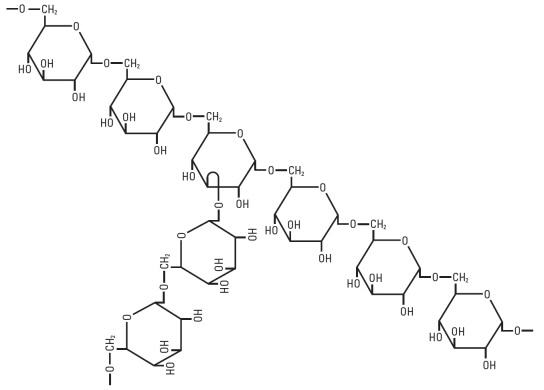
DESCRIPTIONLMD (dextran 40) is a sterile, nonpyrogenic preparation of low molecular weight dextran (average mol. wt. 40,000) in 5% Dextrose Injection or 0.9% Sodium Chloride Injection. It is administered by ...
-
CLINICAL PHARMACOLOGYThe fundamental action of LMD (dextran 40) is the enhancement of blood flow, particularly in the microcirculation. This enhancement is due to: 1. Its primary effect of volume expansion with ...
-
INDICATIONS AND USAGELMD (dextran 40) is indicated for use in the adjunctive treatment of shock or impending shock due to hemorrhage, burns, surgery or other trauma. It is not indicated as a replacement for whole ...
-
CONTRAINDICATIONSLMD (dextran 40) is contraindicated in patients with known hypersensitivity to dextran, in those with marked hemostatic defects of all types (thrombocytopenia, hypofibrinogenemia, etc.) including ...
-
WARNINGSAlthough infrequent, severe and fatal anaphylactoid reactions consisting of marked hypotension or cardiac and respiratory arrest have been reported, most of these reactions have occurred in ...
-
PRECAUTIONSThe possibility of circulatory overload should be kept in mind. Special care should be exercised in patients with impaired renal clearance of dextran. When the risk of pulmonary edema and/or ...
-
ADVERSE REACTIONSAntigenicity of dextrans is directly related to their degree of branching. Since LMD (dextran 40) has a low degree of branching, it is relatively free of antigenic effect. However, a few ...
-
DOSAGE AND ADMINISTRATIONLMD (dextran 40) is administered by I.V. infusion only. Dextran 1 should be administered prior to administration of clinical dextran solutions. 1. In shock, it is suggested that total dosage ...
-
HOW SUPPLIED10% LMD in 5% Dextrose Injection (Dextran 40 in Dextrose Injection, USP) is supplied as follows: Unit of Sale - Concentration Dextran 40 - NDC 0409-7418-03 - Case containing 12 - 500 mL ...
-
REFERENCES1. Richter W. Build-in hapten inhibition of anaphylaxis by the low molecular weight fractions of a B 512 dextran fraction of MW 3400. Int Arch Allergy 1973;45:930 - 2. Lungstrom K-G, Renck H, Hedin ...
-
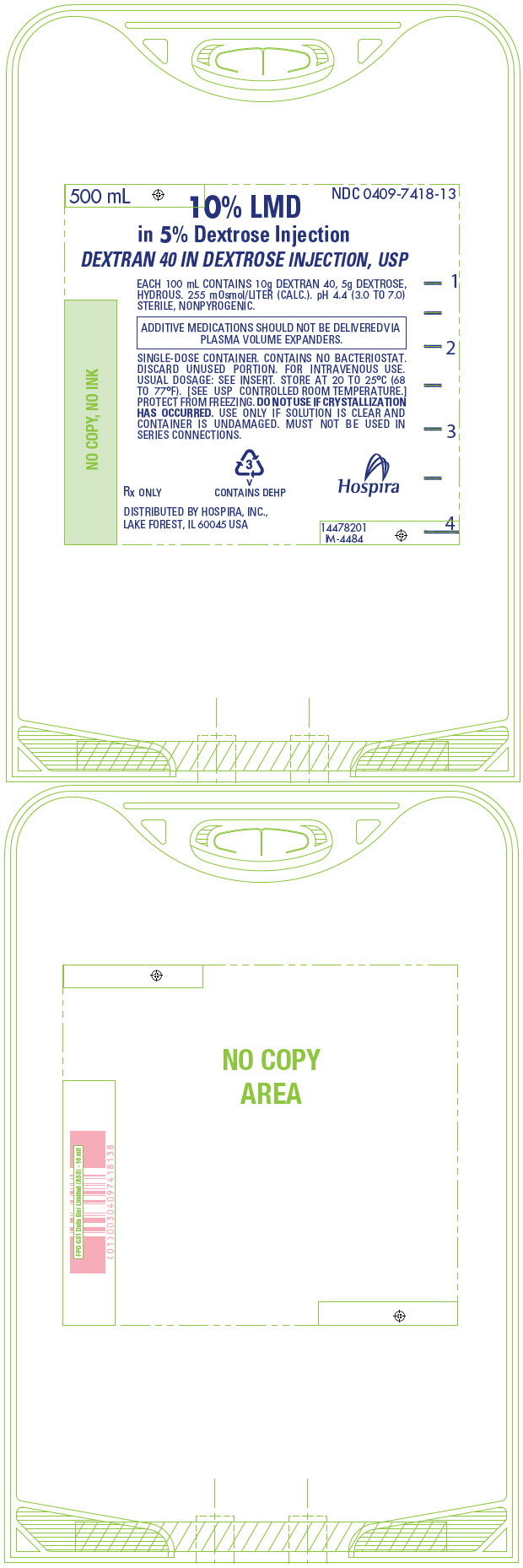
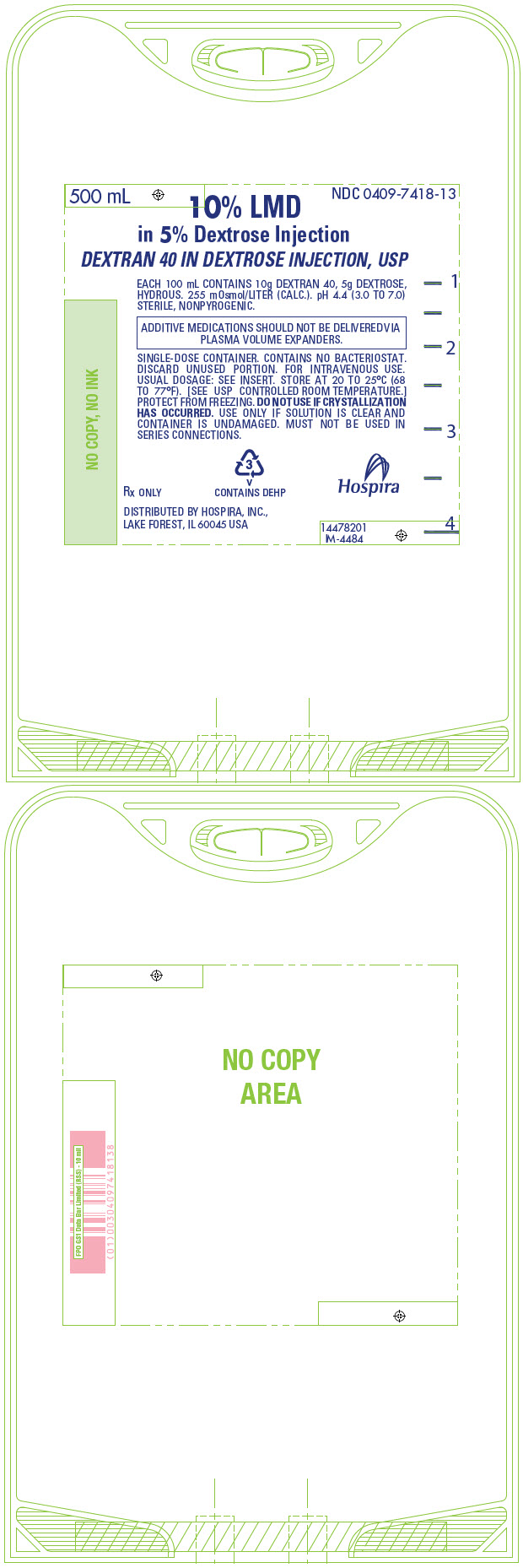
PRINCIPAL DISPLAY PANEL - 500 mL Bag - 7418500 mL - NDC 0409-7418-13 - 10% LMD - in 5% Dextrose Injection - DEXTRAN 40 IN DEXTROSE INJECTION, USP - EACH 100 mL CONTAINS 10g DEXTRAN 40, 5g DEXTROSE, HYDROUS. 255 mOsmol/LITER (CALC.). pH 4.4 (3.0 ...
-
PRINCIPAL DISPLAY PANEL - 500 mL Bag Overwrap - 74182 - HDPE - TO OPEN TEAR AT NOTCH - DO NOT REMOVE FROM OVERWRAP UNTIL READY FOR USE. AFTER REMOVING - THE OVERWRAP, CHECK FOR MINUTE LEAKS BY SQUEEZING CONTAINER - FIRMLY. IF LEAKS ARE FOUND, DISCARD ...
-
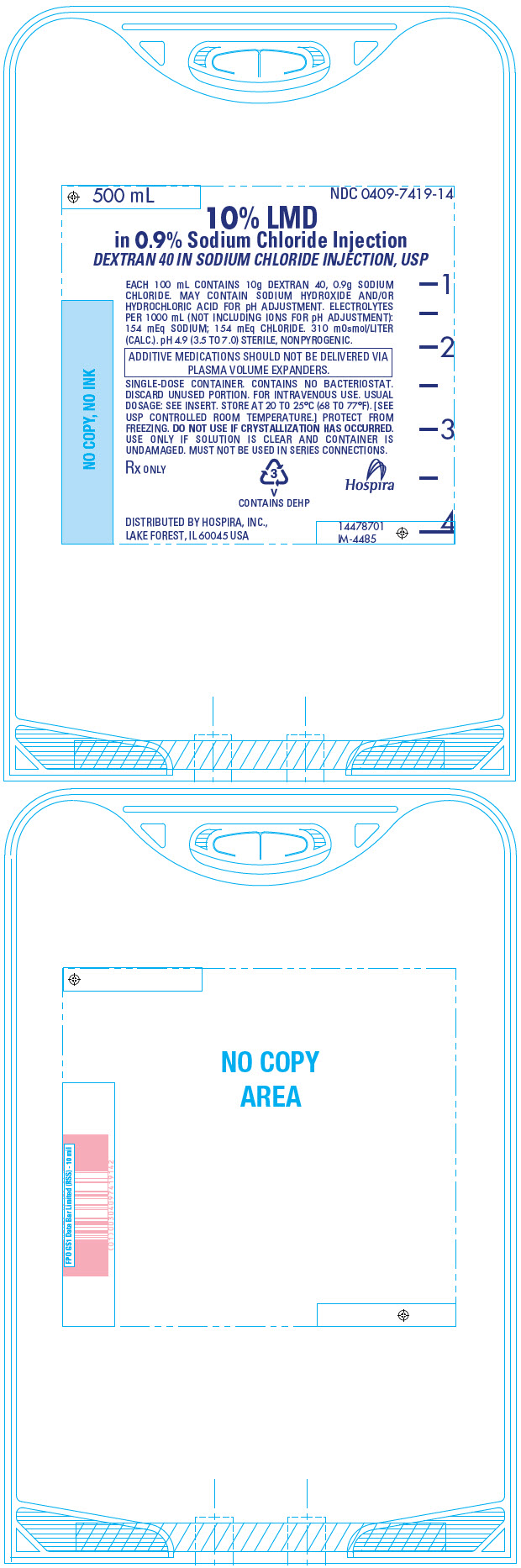
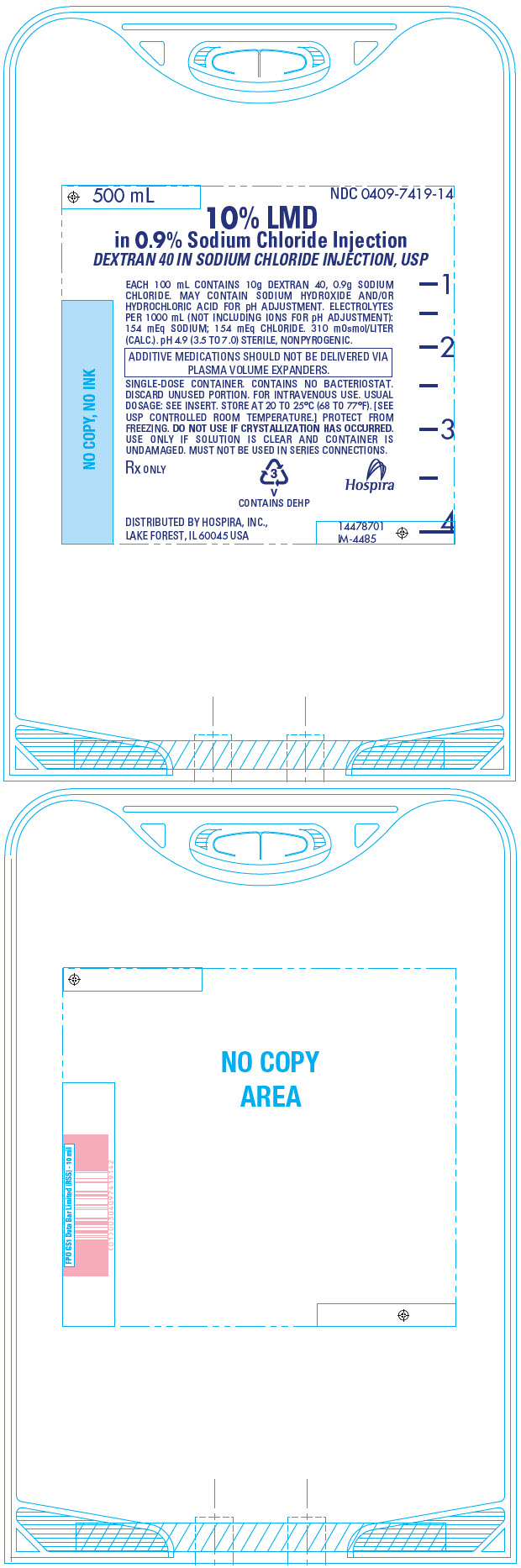
PRINCIPAL DISPLAY PANEL - 500 mL Bag - 7419500 mL - NDC 0409-7419-14 - 10% LMD - in 0.9% Sodium Chloride Injection - DEXTRAN 40 IN SODIUM CHLORIDE INJECTION, USP - EACH 100 mL CONTAINS 10g DEXTRAN 40, 0.9g SODIUM - CHLORIDE. MAY CONTAIN SODIUM ...
-
PRINCIPAL DISPLAY PANEL - 500 mL Bag Overwrap - 74192 - HDPE - TO OPEN TEAR AT NOTCH - DO NOT REMOVE FROM OVERWRAP UNTIL READY FOR USE. AFTER REMOVING - THE OVERWRAP, CHECK FOR MINUTE LEAKS BY SQUEEZING CONTAINER - FIRMLY. IF LEAKS ARE FOUND, DISCARD ...
-
INGREDIENTS AND APPEARANCEProduct Information