Label: METRONIDAZOLE tablet, film coated
- NDC Code(s): 70518-0088-0, 70518-0088-1, 70518-0088-2, 70518-0088-3, view more
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 29300-227
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 26, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of metronidazole tablets and other antibacterial drugs, metronidazole tablets should be used only to treat or ...
-
BOXED WARNING
(What is this?)
Metronidazole has been shown to be carcinogenic in mice and rats (see PRECAUTIONS). Unnecessary use of the drug should be avoided. Its use should be reserved for the conditions described in the INDICATIONS AND USAGEsection below.
Close -
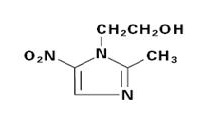
DESCRIPTIONMetronidazole tablets, USP 250 mg or 500 mg is an oral formulation of the synthetic nitroimidazole antimicrobial, 2-methyl-5-nitro-1H-imidazole-1-ethanol, which has the following structural ...
-
CLINICAL PHARMACOLOGYAbsorption - Disposition of metronidazole in the body is similar for both oral and intravenous dosage forms. Following oral administration, metronidazole is well absorbed, with peak plasma ...
-
INDICATIONS AND USAGESymptomatic Trichomoniasis.Metronidazole tablets are indicated for the treatment of - T. vaginalisinfection in females and males when the presence of the trichomonad has been confirmed by ...
-
CONTRAINDICATIONSHypersensitivity - Metronidazole tablet are contraindicated in patients with a prior history of hypersensitivity to metronidazole or other nitroimidazole derivatives. In patients with ...
-
WARNINGSHypersensitivity Reactions - Hypersensitivity reactions including severe cutaneous adverse reactions (SCARs) can be serious and potentially life threatening (see - ADVERSE REACTIONS) ...
-
PRECAUTIONSGeneral - Hepatic Impairment - Patients with hepatic impairment metabolize metronidazole slowly, with resultant accumulation of metronidazole in the plasma. For patients with severe hepatic ...
-
ADVERSE REACTIONSThe following reactions have been reported during treatment with metronidazole: Central Nervous System:The most serious adverse reactions reported in patients treated with metronidazole have ...
-
OVERDOSAGESingle oral doses of metronidazole, up to 15 g, have been reported in suicide attempts and accidental overdoses. Symptoms reported include nausea, vomiting, and ataxia. Oral metronidazole has ...
-
DOSAGE AND ADMINISTRATIONTrichomoniasis: In the Female: One-day treatment −two grams of metronidazole tablets, given either as a single dose or in two divided doses of one gram each, given in the same day ...
-
HOW SUPPLIEDMetronidazole Tablets USP, 500 mg - White colored, capsule shaped, film coated biconvex tablets with 'U' debossed on one side and '227' debossed on other side. NDC: 70518-0088-00 - NDC ...
-
PRINCIPAL DISPLAY PANELDRUG: Metronidazole - GENERIC: Metronidazole - DOSAGE: TABLET, FILM COATED - ADMINSTRATION: ORAL - NDC: 70518-0088-0 - NDC: 70518-0088-1 - NDC: 70518-0088-2 - NDC: 70518-0088-3 - NDC: 70518-0088-4 - NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information






 NADH). Interference is due to the similarity in absorbance peaks of NADH (340 nm) and metronidazole (322 nm) at pH 7
NADH). Interference is due to the similarity in absorbance peaks of NADH (340 nm) and metronidazole (322 nm) at pH 7




