Label: IPRATROPIUM BROMIDE spray
- NDC Code(s): 69238-2017-2
- Packager: Amneal Pharmaceuticals NY LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
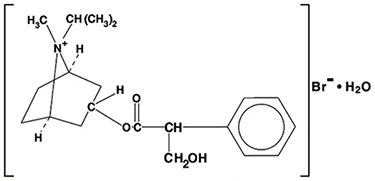
DESCRIPTIONThe active ingredient in Ipratropium Bromide Nasal Solution is ipratropium bromide, USP (as the monohydrate). It is an anticholinergic agent chemically described as 8-azoniabicyclo [3.2.1] octane ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Ipratropium bromide is an anticholinergic (parasympatholytic) agent which, based on animal studies, appears to inhibit vagally-mediated reflexes by antagonizing the action of ...
-
INDICATIONS AND USAGEIpratropium Bromide Nasal Solution 0.06% is indicated for the symptomatic relief of rhinorrhea associated with the common cold or seasonal allergic rhinitis for adults and children age 5 years and ...
-
CONTRAINDICATIONSIpratropium Bromide Nasal Solution 0.06% is contraindicated in patients with a history of hypersensitivity to atropine or its derivatives, or to any of the other ingredients.
-
WARNINGSImmediate hypersensitivity reactions may occur after administration of ipratropium bromide, as demonstrated by urticaria, angioedema, rash, bronchospasm and oropharyngeal edema. If such a reaction ...
-
PRECAUTIONSGeneral - Effects Seen with Anticholinergic Drugs: Ipratropium Bromide Nasal Solution 0.06% should be used with caution in patients with narrow-angle glaucoma, prostatic hyperplasia, or bladder ...
-
ADVERSE REACTIONSAdverse reaction information on Ipratropium Bromide Nasal Solution 0.06% in patients with the common cold was derived from two multicenter, vehicle-controlled clinical trials involving 1,276 ...
-
OVERDOSAGEAcute overdosage by intranasal administration is unlikely since ipratropium bromide is not well absorbed systemically after intranasal or oral administration. Following administration of a 20 mg ...
-
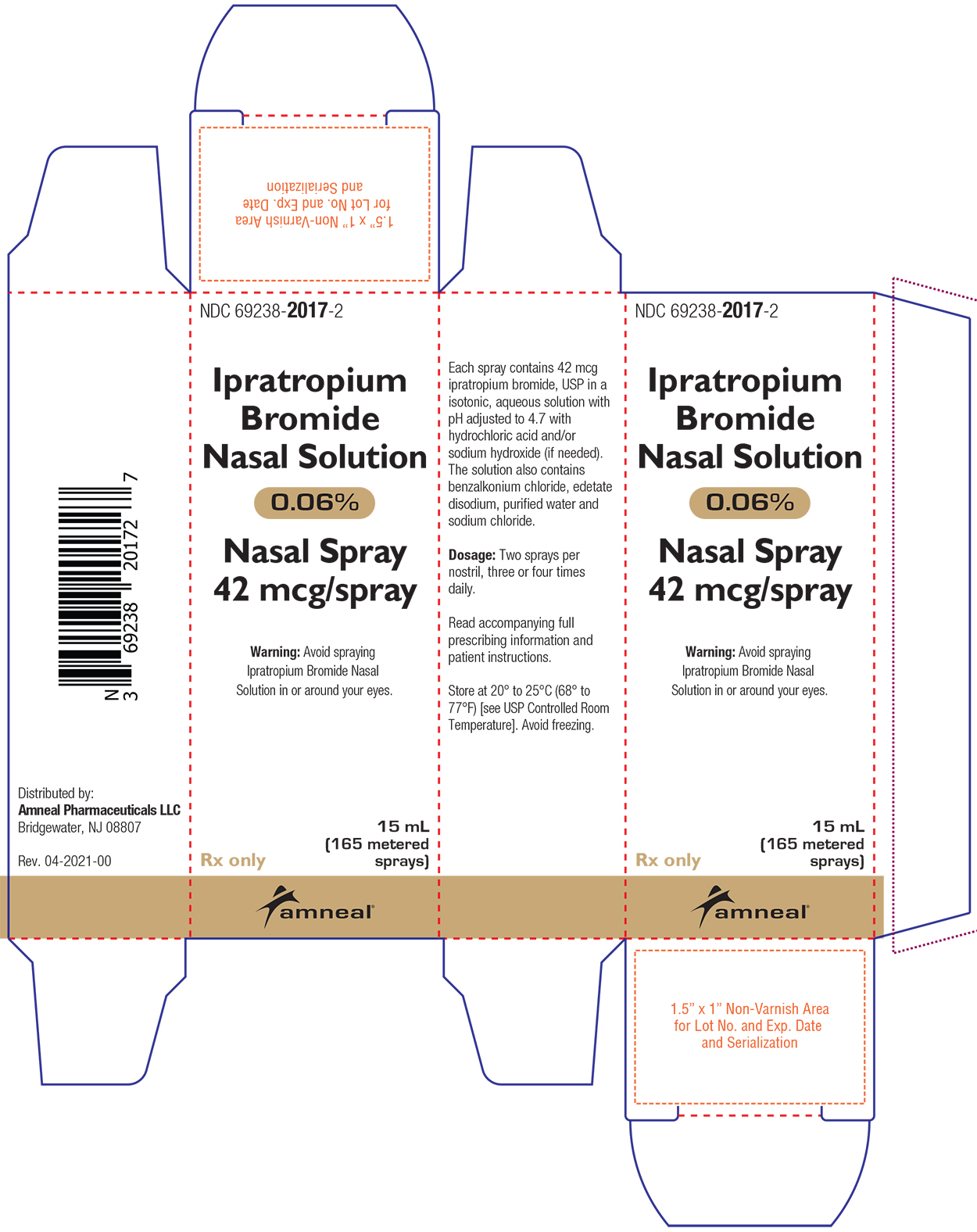
DOSAGE AND ADMINISTRATIONFor Symptomatic Relief of Rhinorrhea Associated with the Common Cold - The recommended dose of Ipratropium Bromide Nasal Solution 0.06% is two sprays (84 mcg) per nostril three or four times daily ...
-
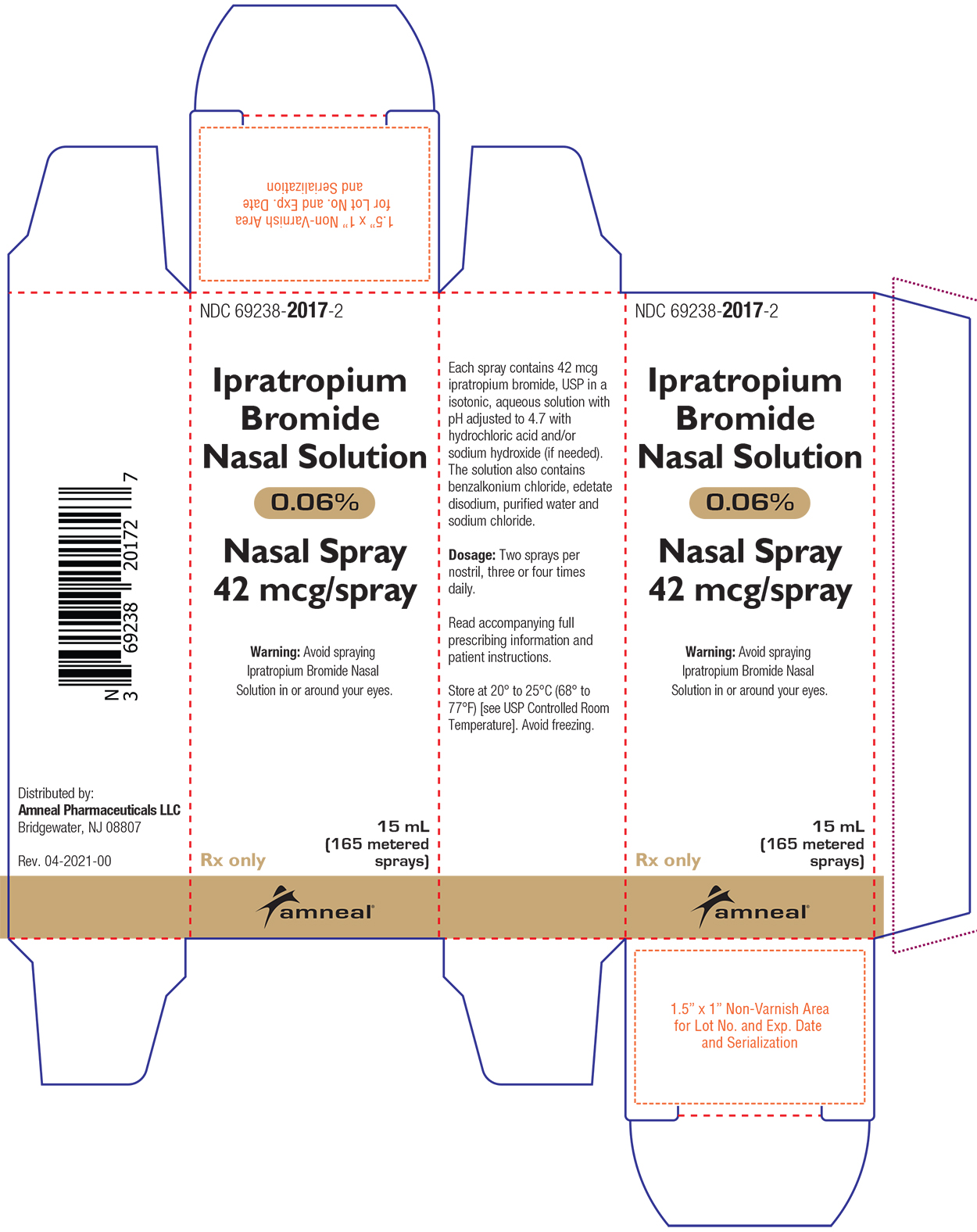
HOW SUPPLIEDIpratropium Bromide Nasal Solution 0.06% is a clear, colorless solution supplied in a white high density polyethylene (HDPE) bottle fitted with a white colored metered nasal spray pump ...
-
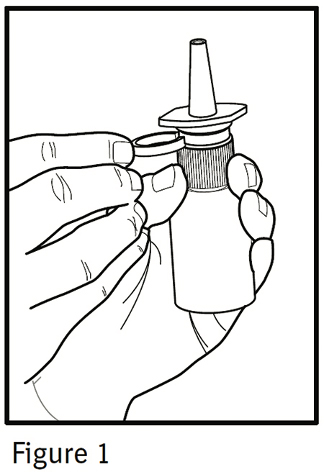
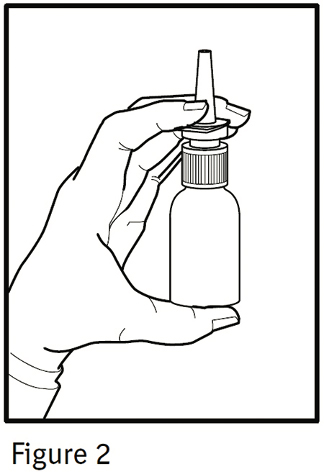
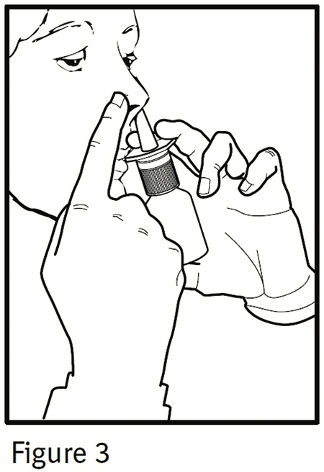
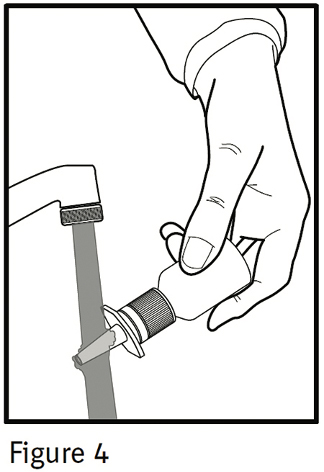
PATIENT'S INSTRUCTIONS FOR USEIPRATROPIUM BROMIDE Nasal Solution 0.06% NASAL SPRAY 42 mcg/spray - Read complete instructions carefully before using. In order to ensure proper dosing, do not attempt to change the size of ...
-
PRINCIPAL DISPLAY PANEL
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information