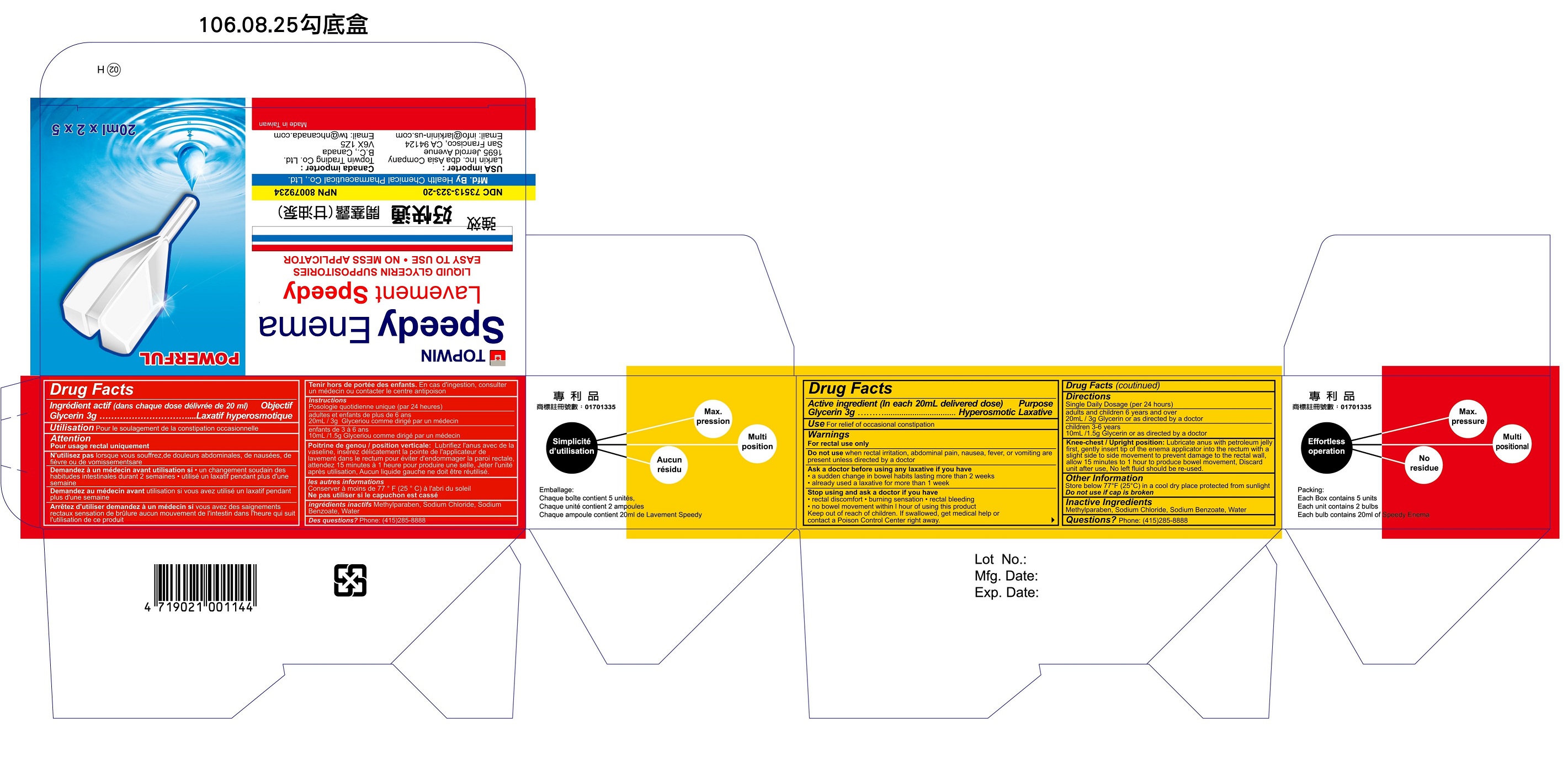
Label: TOPWIN SPEEDY ENEMA- glycerin enema
- NDC Code(s): 73513-323-20
- Packager: Health Chemical Pharmaceutical Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- Do not use
- Ask a doctor before using any laxative if you have
- Stop using and ask a doctor if you have
- Keep out of reach of children
-
Directions
- Single Daily Usage (per 24 hours)
- Adults and children, 6 years and over: 20 ml / 3g Glycerin or as directed by a doctor
- Children 3-6 years: 10 ml / 1.5g Glycerin or as directed by a doctor
- Knee-chest / Upright position: Lubricate anus with petroleum jelly first, gently insert tip of the enema applicator into the rectum with a single side to side movement to prevent damage to the rectal wall, allow 15 minutes to 1 hour to produce bowel movement. Discard unit after use. No left fluid should be re-used.
- Other Information
- Inactive Ingredients
- Questions?
- Topwin Speedy Enema NDC 73513-323-20
-
INGREDIENTS AND APPEARANCE
TOPWIN SPEEDY ENEMA
glycerin enemaProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73513-323 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 3 g in 100 mL Inactive Ingredients Ingredient Name Strength METHYLPARABEN (UNII: A2I8C7HI9T) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM BENZOATE (UNII: OJ245FE5EU) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73513-323-20 5 in 1 BOX 05/28/2020 1 2 in 1 PACKET 1 20 mL in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 05/28/2020 Labeler - Health Chemical Pharmaceutical Co., Ltd (656344421) Registrant - Health Chemical Pharmaceutical Co., Ltd (656344421) Establishment Name Address ID/FEI Business Operations Health Chemical Pharmaceutical Co., Ltd 656344421 manufacture(73513-323)