Label: ACETAMINOPHEN AND CODEINE PHOSPHATE tablet
-
NDC Code(s):
49999-060-00,
49999-060-01,
49999-060-06,
49999-060-10, view more49999-060-12, 49999-060-15, 49999-060-20, 49999-060-24, 49999-060-30, 49999-060-50, 49999-060-60, 49999-060-90
- Packager: Quality Care Products, LLC
- This is a repackaged label.
- Source NDC Code(s): 0406-0484
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
HepatotoxicityAcetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and often involve more than one acetaminophen-containing product.
-
DESCRIPTION
Acetaminophen and codeine phosphate is supplied in tablet form for oral administration.
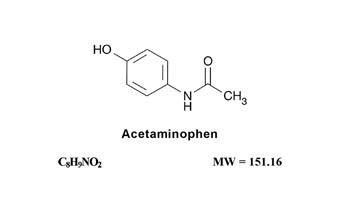
Acetaminophen, 4'-hydroxyacetanilide, a slightly bitter, white, odorless, crystalline powder, is a non-opiate, non-salicylate analgesic and antipyretic. It has the following structural formula:
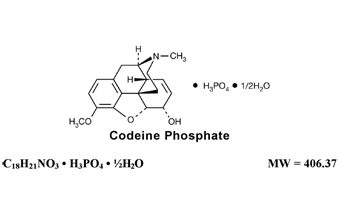
Codeine phosphate, 7,8-didehydro-4,5α-epoxy-3-methoxy-17-methylmorphinan-6α-ol phosphate (1:1) (salt) hemihydrate, a white crystalline powder, is a narcotic analgesic and antitussive. It has the following structural formula:
Each Acetaminophen and Codeine Phosphate Tablet USP (300 mg/30 mg) contains:
Acetaminophen USP . . . . . . . . . . . . . 300 mgCodeine Phosphate USP . . . . . . . . . . . . . 30 mg
Each Acetaminophen and Codeine Phosphate Tablet USP (300 mg/60 mg) contains:
Acetaminophen USP . . . . . . . . . . . . . 300 mgCodeine Phosphate USP . . . . . . . . . . . . . 60 mg
In addition, each Acetaminophen and Codeine Phosphate Tablet USP contains the following inactive ingredients: crospovidone, magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch, stearic acid.
-
CLINICAL PHARMACOLOGY
This product combines the analgesic effects of a centrally acting analgesic, codeine, with a peripherally acting analgesic, acetaminophen.
Pharmacokinetics
The behavior of the individual components is described below.
Codeine – Codeine is readily absorbed from the gastrointestinal tract. It is rapidly distributed from the intravascular spaces to the various body tissues, with preferential uptake by parenchymatous organs such as the liver, spleen and kidney. Codeine crosses the blood-brain barrier, and is found in fetal tissue and breast milk. The plasma concentration does not correlate with brain concentration or relief of pain; however, codeine is not bound to plasma proteins and does not accumulate in body tissues.
The plasma half-life is about 2.9 hours. The elimination of codeine is primarily via the kidneys, and about 90% of an oral dose is excreted by the kidneys within 24 hours of dosing. The urinary secretion products consist of free and glucuronide conjugated codeine (about 70%), free and conjugated norcodeine (about 10%), free and conjugated morphine (about 10%), normorphine (4%), and hydrocodone (1%). The remainder of the dose is excreted in the feces.
At therapeutic doses, the analgesic effect reaches a peak within 2 hours and persists between 4 and 6 hours.
See OVERDOSAGE for toxicity information.
Acetaminophen – Acetaminophen is rapidly absorbed from the gastrointestinal tract and is distributed throughout most body tissues. The plasma half-life is 1.25 to 3 hours, but may be increased by liver damage and following overdosage. Elimination of acetaminophen is principally by liver metabolism (conjugation) and subsequent renal excretion of metabolites. Approximately 85% of an oral dose appears in the urine within 24 hours of administration, most as the glucuronide conjugate, with small amounts of other conjugates and unchanged drug.
See OVERDOSAGE for toxicity information.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
SPL UNCLASSIFIED SECTION
Hepatotoxicity
Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and often involve more than one acetaminophen-containing product. The excessive intake of acetaminophen may be intentional to cause self-harm or unintentional as patients attempt to obtain more pain relief or unknowingly take other acetaminophen-containing products.
The risk of acute liver failure is higher in individuals with underlying liver disease and in individuals who ingest alcohol while taking acetaminophen.
Instruct patients to look for acetaminophen or APAP on package labels and not to use more than one product that contains acetaminophen. Instruct patients to seek medical attention immediately upon ingestion of more than 4000 milligrams of acetaminophen per day, even if they feel well.
-
SPL UNCLASSIFIED SECTION
Hypersensitivity/anaphylaxis
There have been post-marketing reports of hypersensitivity and anaphylaxis associated with use of acetaminophen. Clinical signs included swelling of the face, mouth, and throat, respiratory distress, urticaria, rash, pruritus, and vomiting. There were infrequent reports of life-threatening anaphylaxis requiring emergency medical attention. Instruct patients to discontinue acetaminophen and codeine phosphate tablets USP immediately and seek medical care if they experience these symptoms. Do not prescribe acetaminophen and codeine phosphate tablets USP for patients with acetaminophen allergy.
In the presence of head injury or other intracranial lesions, the respiratory depressant effects of codeine and other narcotics may be markedly enhanced, as well as their capacity for elevating cerebrospinal fluid pressure. Narcotics also produce other CNS depressant effects, such as drowsiness, that may further obscure the clinical course of the patients with head injuries.
Codeine or other narcotics may obscure signs on which to judge the diagnosis or clinical course of patients with acute abdominal conditions.
Codeine is habit-forming and potentially abusable. Consequently, the extended use of this product is not recommended.
-
PRECAUTIONS
General
Acetaminophen and codeine phosphate tablets should be prescribed with caution in certain special risk patients, such as the elderly or debilitated, and those with severe impairment of renal or hepatic function, head injuries, elevated intracranial pressure, acute abdominal conditions, hypothyroidism, urethral stricture, Addison's disease, or prostatic hypertrophy.
Ultra-rapid Metabolizers of Codeine - Some individuals may be ultra-rapid metabolizers due to a specific CYP2D6*2x2 genotype. These individuals convert codeine into its active metabolite, morphine, more rapidly and completely than other people. This rapid conversion results in higher than expected serum morphine levels. Even at labeled dosage regiments, individuals who are ultra-rapid metabolizers may experience overdose symptoms such as extreme sleepiness, confusion or shallow breathing.
The prevalence of this CYP2D6 phenotype varies widely and has been estimated at 0.5 to 1% in Chinese and Japanese, 0.5 to 1% in Hispanics, 1 to 10% in Caucasians, 3% in African Americans, and 16 to 28% in North Africans, Ethiopians and Arabs. Data is not available for other ethnic groups.
When physicians prescribe codeine-containing drugs, they should choose the lowest effective dose for the shortest period of time and should inform their patients about these risks and the signs of morphine overdose (see PRECAUTIONS, Nursing Mothers).
Information for Patients/Caregivers
- Do not take acetaminophen and codeine phosphate tablets USP if you are allergic to any of its ingredients.
- If you develop signs of allergy such as a rash or difficulty breathing stop taking acetaminophen and codeine phosphate tablets USP and contact your healthcare provider immediately.
- Do not take more than 4000 milligrams of acetaminophen per day. Call your doctor if you took more than the recommended dose.
Codeine may impair mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. Such tasks should be avoided while taking this product.
Alcohol and other CNS depressants may produce an additive CNS depression when taken with this combination product, and should be avoided.
Codeine is habit-forming. Patients should take the drug only for as long as it is prescribed, in the amounts prescribed, and no more frequently than prescribed.
Caution patients that some people have a variation in a liver enzyme and change codeine into morphine more rapidly and completely than other people. These people are ultra-rapid metabolizers and are more likely to have higher-than-normal levels of morphine in their blood after taking codeine which can result in overdose symptoms such as extreme sleepiness, confusion, or shallow breathing. In most cases, it is unknown if someone is an ultra-rapid codeine metabolizer.
Nursing mothers taking codeine can also have higher morphine levels in their breast milk if they are ultra-rapid metabolizers. These higher levels of morphine in breast milk may lead to life-threatening or fatal side effects in nursing babies. Instruct nursing mothers to watch for signs of morphine toxicity in their infants including increased sleepiness (more than usual), difficulty breastfeeding, breathing difficulties, or limpness. Instruct nursing mothers to talk to the baby's doctor immediately if they notice these signs and, if they cannot reach the doctor right away, to take the baby to an emergency room or call 911 (or local emergency services).
Laboratory Tests
In patients with severe hepatic or renal disease, effects of therapy should be monitored with serial liver and/or renal function tests.
Drug Interactions
This drug may enhance the effects of other narcotic analgesics, alcohol, general anesthetics, tranquilizers such as chlordiazepoxide, sedative-hypnotics, or other CNS depressants, causing increased CNS depression.
Drug/Laboratory Test Interactions
Codeine may increase serum amylase levels.
Acetaminophen may produce false positive test results for urinary 5-hydroxyindoleacetic acid.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No adequate studies have been conducted in animals to determine whether acetaminophen and codeine have a potential for carcinogenesis or mutagenesis. No adequate studies have been conducted in animals to determine whether acetaminophen has a potential for impairment of fertility.
Acetaminophen and codeine have been found to have no mutagenic potential using the Ames Salmonella-Microsomal Activation test, the Basc test on Drosophila germ cells, and the Micronucleus test on mouse bone marrow.
Pregnancy
Teratogenic Effects. Pregnancy Category C
Codeine – A study in rats and rabbits reported no teratogenic effect of codeine administered during the period of organogenesis in doses ranging from 5 to 120 mg/kg. In the rat, doses at the 120 mg/kg level, in the toxic range for the adult animal, were associated with an increase in embryo resorption at the time of implantation. In another study a single 100 mg/kg dose of codeine administered to pregnant mice reportedly resulted in delayed ossification in the offspring.
There are no adequate and well-controlled studies in pregnant women. Acetaminophen and codeine phosphate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Labor and Delivery
Narcotic analgesics cross the placental barrier. The closer to delivery and the larger the dose used, the greater the possibility of respiratory depression in the newborn. Narcotic analgesics should be avoided during labor if delivery of a premature infant is anticipated. If the mother has received narcotic analgesics during labor, newborn infants should be observed closely for signs of respiratory depression. Resuscitation may be required (see OVERDOSAGE). The effect of codeine, if any, on the later growth, development, and functional maturation of the child is unknown.
Nursing Mothers
Acetaminophen is excreted in breast milk in small amounts, but the significance of its effects on nursing infants is not known. Because of the potential for serious adverse reactions in nursing infants from acetaminophen, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Codeine is secreted into human milk. In women with normal codeine metabolism (normal CYP2D6 activity), the amount of codeine secreted into human milk is low and dose-dependent. Despite the common use of codeine products to manage postpartum pain, reports of adverse events in infants are rare. However, some women are ultra-rapid metabolizers of codeine. These women achieve higher-than-expected serum levels of codeine's active metabolite, morphine, leading to higher-than-expected levels of morphine in breast milk and potentially dangerously high serum morphine levels in their breastfed infants. Therefore, maternal use of codeine can potentially lead to serious adverse reactions, including death, in nursing infants.
The prevalence of this CYP2D6 phenotype varies widely and has been estimated at 0.5 to 1% in Chinese and Japanese, 0.5 to 1% in Hispanics, 1 to 10% in Caucasians, 3% in African Americans, and 16 to 28% in North Africans, Ethiopians and Arabs. Data is not available for other ethnic groups.
The risk of infant exposure to codeine and morphine through breast milk should be weighed against the benefits of breastfeeding for both the mother and baby. Caution should be exercised when codeine is administered to a nursing woman. If a codeine containing product is selected, the lowest dose should be prescribed for the shortest period of time to achieve the desired clinical effect. Mothers using codeine should be informed about when to seek immediate medical care and how to identify the signs and symptoms of neonatal toxicity, such as drowsiness or sedation, difficulty breastfeeding, breathing difficulties, and decreased tone, in their baby. Nursing mothers who are ultra-rapid metabolizers may also experience overdose symptoms such as extreme sleepiness, confusion or shallow breathing. Prescribers should closely monitor mother-infant pairs and notify treating pediatricians about the use of codeine during breastfeeding (see PRECAUTIONS, General, Ultra-rapid Metabolizers of Codeine).
-
ADVERSE REACTIONS
The most frequently reported adverse reactions are drowsiness, lightheadedness, dizziness, sedation, shortness of breath, nausea and vomiting. These effects seem to be more prominent in ambulatory than in non-ambulatory patients, and some of these adverse reactions may be alleviated if the patient lies down. Other adverse reactions include allergic reactions, euphoria, dysphoria, constipation, abdominal pain, pruritus, rash, thrombocytopenia, agranulocytosis.
At higher doses, codeine has most of the disadvantages of morphine including respiratory depression.
-
DRUG ABUSE AND DEPENDENCE
Controlled Substance
Acetaminophen and codeine phosphate tablets are classified as a Schedule III controlled substance.
Abuse and Dependence
Codeine can produce drug dependence of the morphine type and, therefore, has the potential for being abused. Psychological dependence, physical dependence, and tolerance may develop upon repeated administration and it should be prescribed and administered with the same degree of caution appropriate to the use of other oral narcotic medications.
-
OVERDOSAGE
Following an acute overdosage, toxicity may result from codeine or acetaminophen.
Signs and Symptoms
Toxicity from codeine poisoning includes the opioid triad of pinpoint pupils, depression of respiration, and loss of consciousness. Convulsions may occur.
In acetaminophen overdosage, dose-dependent, potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma and coagulation defects may also occur. Early symptoms following a potentially hepatotoxic overdose may include nausea, vomiting, diaphoresis and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion.
Treatment
A single or multiple drug overdose with acetaminophen and codeine is a potentially lethal polydrug overdose, and consultation with a regional poison control center is recommended. Immediate treatment includes support of cardiorespiratory function and measures to reduce drug absorption.
Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated. Assisted or controlled ventilation should also be considered. For respiratory depression due to overdosage or unusual sensitivity to codeine, parenteral naloxone is a specific and effective antagonist.
Gastric decontamination with activated charcoal should be administered just prior to N-acetylcysteine (NAC) to decrease systemic absorption if acetaminophen ingestion is known or suspected to have occurred within a few hours of presentation. Serum acetaminophen levels should be obtained immediately if the patient presents 4 hours or more after ingestion to assess potential risk of hepatotoxicity; acetaminophen levels drawn less than 4 hours post-ingestion may be misleading. To obtain the best possible outcome, NAC should be administered as soon as possible where impending or evolving liver injury is suspected. Intravenous NAC may be administered when circumstances preclude oral administration.
Vigorous supportive therapy is required in severe intoxication. Procedures to limit the continuing absorption of the drug must be readily performed since the hepatic injury is dose dependent and occurs early in the course of intoxication.
-
DOSAGE AND ADMINISTRATION
Dosage should be adjusted according to severity of pain and response of the patient.
The usual adult dosage for tablets is:
Single Doses (Range) Maximum 24 Hour Dose Codeine Phosphate 15 mg to 60 mg 360 mg Acetaminophen 300 mg to 1000 mg 4000 mg The usual dose of codeine phosphate in children is 0.5 mg/kg.
Doses may be repeated up to every 4 hours.
The prescriber must determine the number of tablets per dose, and the maximum number of tablets per 24 hours based upon the above dosage guidance. This information should be conveyed in the prescription.
It should be kept in mind, however, that tolerance to codeine can develop with continued use and that the incidence of untoward effects is dose related. Adult doses of codeine higher than 60 mg fail to give commensurate relief of pain but merely prolong analgesia and are associated with an appreciably increased incidence of undesirable side effects. Equivalently high doses in children would have similar effects.
-
HOW SUPPLIED
Each Acetaminophen and Codeine Phosphate Tablet USP 300 mg/15 mg tablet contains acetaminophen 300 mg and codeine phosphate 15 mg. It is available as a round, white to off-white tablet debossed with "2" on one side and an M on the other side.
Each Acetaminophen and Codeine Phosphate Tablet USP 300 mg/30 mg tablet contains acetaminophen 300 mg and codeine phosphate 30 mg. It is available as a round, white to off-white tablet debossed with "3" on one side and an M on the other side.
49999-060-06
49999-060-15
49999-060-12
49999-060-10
49999-060-20
49999-060-24
49999-060-30
49999-060-50
49999-060-60
49999-060-90
49999-060-00
49999-060-01
Each Acetaminophen and Codeine Phosphate Tablet USP 300 mg/60 mg tablet contains acetaminophen 300 mg and codeine phosphate 60 mg. It is available as a round, white to off-white tablet debossed with "4" on one side and an M on the other side.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Dispense in tight, light-resistant container as defined in the USP.
 is a trademark of Mallinckrodt Inc.
is a trademark of Mallinckrodt Inc.Mallinckrodt Inc.
Hazelwood, MO 63042 USARev 06/2011
Mallinckrodt
COVIDIEN™ - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN AND CODEINE PHOSPHATE
acetaminophen and codeine phosphate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49999-060(NDC:0406-0484) Route of Administration ORAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 300 mg CODEINE PHOSPHATE (UNII: GSL05Y1MN6) (CODEINE ANHYDROUS - UNII:UX6OWY2V7J) CODEINE PHOSPHATE 30 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (UNII: 2S7830E561) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white (to off-white) Score no score Shape ROUND Size 11mm Flavor Imprint Code 3;M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49999-060-06 6 in 1 BOTTLE; Type 0: Not a Combination Product 12/16/2011 12/31/2016 2 NDC:49999-060-10 10 in 1 BOTTLE; Type 0: Not a Combination Product 09/14/2011 12/31/2016 3 NDC:49999-060-12 12 in 1 BOTTLE; Type 0: Not a Combination Product 09/14/2011 12/31/2016 4 NDC:49999-060-15 15 in 1 BOTTLE; Type 0: Not a Combination Product 09/14/2011 12/31/2016 5 NDC:49999-060-20 20 in 1 BOTTLE; Type 0: Not a Combination Product 09/14/2011 12/31/2020 6 NDC:49999-060-24 24 in 1 BOTTLE; Type 0: Not a Combination Product 09/14/2011 12/31/2020 7 NDC:49999-060-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 09/14/2011 02/28/2026 8 NDC:49999-060-50 50 in 1 BOTTLE; Type 0: Not a Combination Product 09/14/2011 12/31/2020 9 NDC:49999-060-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 09/14/2011 09/30/2026 10 NDC:49999-060-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 09/14/2011 12/31/2023 11 NDC:49999-060-00 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/14/2011 12/31/2020 12 NDC:49999-060-01 120 in 1 BOTTLE; Type 0: Not a Combination Product 09/14/2011 12/31/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040419 09/14/2011 09/30/2026 Labeler - Quality Care Products, LLC (831276758) Establishment Name Address ID/FEI Business Operations Quality Care Products, LLC 831276758 repack(49999-060)


