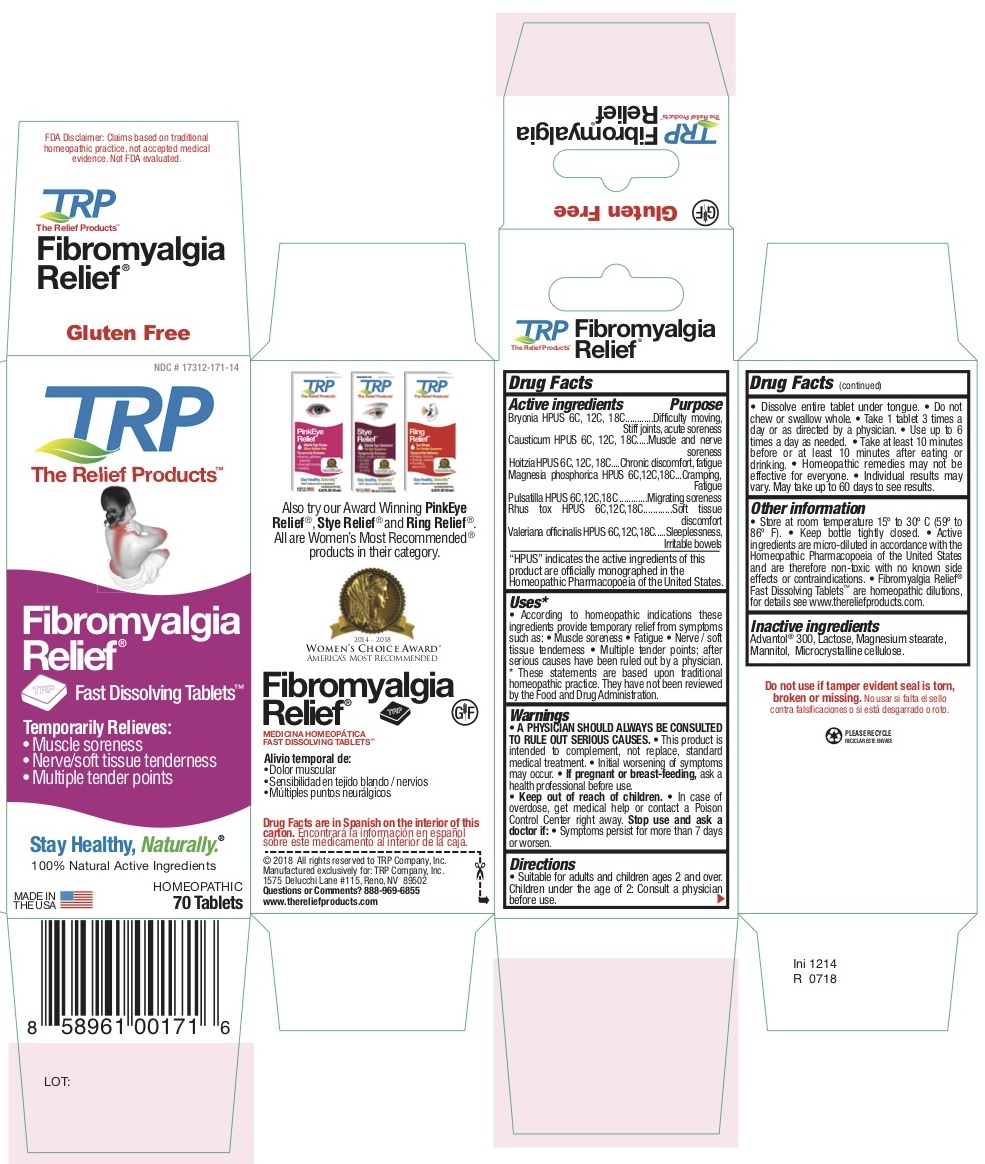
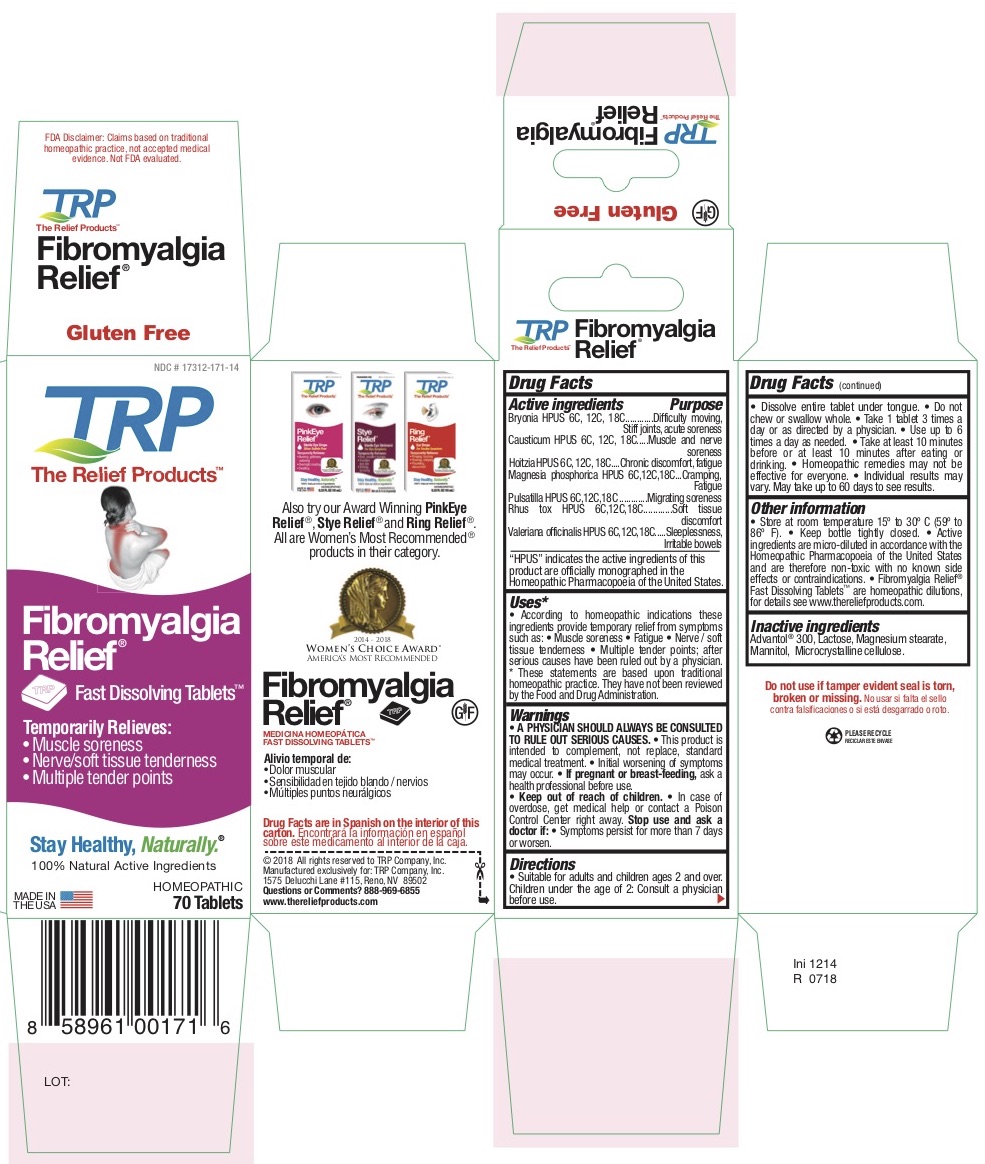
Label: TEMPORARY FIBROMYALGIA PAIN AND DISCOMFORT RELIEF- atropa belladonna - bryonia alba root - causticum - phosphorus - strychnos nux-vomica seed - pulsatilla vulgaris - toxicodendron pubescens leaf - tablet, orally disintegrating
- NDC Code(s): 17312-016-14
- Packager: TRP Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
PURPOSE
Belladonna HPUS - Chronic discomfort, Fatigue
Bryonia HPUS - Difficulty moving, Stiff joints, Acute soreness
Causticum HPUS - Muscle and nerve soreness
Magnesia Phosphorica HPUS - Cramping, Fatigue
Nux Vomica HPUS - Sleeplessness, Irritable bowels
Pulsatilla HPUS - Migrating soreness
Rhus Tox HPUS - Soft tissue discomfort -
Uses
According to homeopathic indications these ingredients provide temporary relief from symptoms of Fibromyalgia such as: • Muscle soreness • Fatigue • Nerve / Soft tissue tenderness • Multiple tender points after diagnosis by a physician.
* These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
- Keep out of reach of children
- PREGNANCY OR BREAST FEEDING
-
Directions
- Suitable for adults and children 12 years and above.
- Dissolve entire tablet under tongue.
- Do not chew or swallow whole.
- Take 1 tablet 3 times a day or as directed by a physician.
- Use up to 6 times a day as needed.
- Take at least 10 minutes before or at least 10 minutes after eating or drinking.
- Children under the age of 12: consult a physician before use.
-
Other information
There are no known contraindications.
- Active ingredients are micro-diluted in accordance with the Homeopathic Pharmacopoeia of the United States and are therefore non-toxic with no known side cool dark location. Temporary Fibromyalgia Pain and Discomfort Relief® Fast Dissolving TabletsTM are homeopathic dilutions, for details see www.thereliefproducts.com.
- Inactive Ingredients
- Do not use if tamper evident seal is torn, broken or missing.
- QUESTIONS
- Do not use
- Stop use
-
Warning
USE ONLY AFTER DIAGNOSIS BY A PHYSICIAN.
- This product is intended to complement, not replace, standard medical treatment.
- Initial worsening of symptoms may occur.
- A physician should always be consulted regarding Fibromyalgia to rule out serious causes.
- In case of overdose, get medical help or contact a Poison Control Center right away.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TEMPORARY FIBROMYALGIA PAIN AND DISCOMFORT RELIEF
atropa belladonna - bryonia alba root - causticum - phosphorus - strychnos nux-vomica seed - pulsatilla vulgaris - toxicodendron pubescens leaf - tablet, orally disintegratingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17312-016 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 6 [hp_C] BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 6 [hp_C] CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 6 [hp_C] PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 6 [hp_C] STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 6 [hp_C] PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 6 [hp_C] TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 6 [hp_C] Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SORBITOL (UNII: 506T60A25R) CROSPOVIDONE (UNII: 68401960MK) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) COPOVIDONE (UNII: D9C330MD8B) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape DIAMOND Size 13mm Flavor Imprint Code TRP Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17312-016-14 1 in 1 PACKAGE 12/01/2011 1 70 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/01/2011 Labeler - TRP Company (105185719)