Label: LISDEXAMFETAMINE DIMESYLATE tablet, chewable
-
NDC Code(s):
0406-5124-01,
0406-5125-01,
0406-5126-01,
0406-5127-01, view more0406-5128-01, 0406-5129-01
- Packager: SpecGx LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LISDEXAMFETAMINE DIMESYLATE CHEWABLE TABLETS safely and effectively. See full prescribing information for LISDEXAMFETAMINE ...These highlights do not include all the information needed to use LISDEXAMFETAMINE DIMESYLATE CHEWABLE TABLETS safely and effectively. See full prescribing information for LISDEXAMFETAMINE DIMESYLATE CHEWABLE TABLETS.
LISDEXAMFETAMINE DIMESYLATE chewable tablets, for oral use, CII
Initial U.S. Approval: 2007
WARNING: ABUSE, MISUSE, AND ADDICTION
See full prescribing information for complete boxed warning.
Lisdexamfetamine dimesylate chewable tablets have a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including lisdexamfetamine dimesylate chewable tablets, can result in overdose and death. (5.1, 9.2, 10):
-
Before prescribing lisdexamfetamine dimesylate chewable tablets, assess each patient’s risk for abuse, misuse, and addiction.
-
Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug.
-
Throughout treatment, reassess each patient’s risk and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Lisdexamfetamine dimesylate chewable tablets are a central nervous system (CNS) stimulant indicated for the treatment of (1):
- Attention Deficit Hyperactivity Disorder (ADHD) in adults and pediatric patients 6 years and older
- Moderate to severe binge eating disorder (BED) in adults
Limitations of Use:
- Pediatric patients with ADHD younger than 6 years of age experienced more long-term weight loss than patients 6 years and older. (8.4)
- Lisdexamfetamine dimesylate chewable tablets are not indicated for weight loss. Use of other sympathomimetic drugs for weight loss has been associated with serious cardiovascular adverse events. The safety and effectiveness of lisdexamfetamine dimesylate chewable tablets for the treatment of obesity have not been established. (5.2)
DOSAGE AND ADMINISTRATION
Indicated Population
Initial Dose
Titration Schedule
Recommended Dose
Maximum Dose
ADHD (Adults and pediatric patients 6 years and older) (2.2)
30 mg every morning
10 mg or 20 mg weekly
30 mg to 70 mg per day
70 mg per day
BED (Adults) (2.3)
30 mg every morning
20 mg weekly
50 mg to 70 mg per day
70 mg per day
DOSAGE FORMS AND STRENGTHS
Chewable tablets: 10 mg, 20 mg, 30 mg, 40 mg, 50 mg, 60 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
-
Risks to Patients with Serious Cardiac Disease: Avoid use in patients with known structural cardiac abnormalities, cardiomyopathy, serious cardiac arrhythmia, coronary artery disease, or other serious cardiac disease. (5.2)
-
Increased Blood Pressure and Heart Rate: Monitor blood pressure and pulse. (5.3)
-
Psychiatric Adverse Reactions: Prior to initiating lisdexamfetamine dimesylate chewable tablets, screen patients for risk factors for developing a manic episode. If new psychotic or manic symptoms occur, consider discontinuing lisdexamfetamine dimesylate chewable tablets. (5.4)
-
Long-Term Suppression of Growth in Pediatric Patients: Closely monitor growth (height and weight) in pediatric patients. Pediatric patients not growing or gaining height or weight as expected may need to have their treatment interrupted. (5.5)
-
Peripheral Vasculopathy, including Raynaud’s Phenomenon: Careful observation for digital changes is necessary during lisdexamfetamine dimesylate chewable tablets treatment. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for patients who develop signs or symptoms of peripheral vasculopathy. (5.6)
-
Serotonin Syndrome: Increased risk when co-administered with serotonergic agents (e.g., SSRIs, SNRIs, triptans), but also during overdosage situations. If it occurs, discontinue lisdexamfetamine dimesylate chewable tablets and initiate supportive treatment. (4, 5.7, 10)
-
Motor and Verbal Tics, and Worsening of Tourette’s Syndrome: Before initiating lisdexamfetamine dimesylate chewable tablets, assess the family history and clinically evaluate patients for tics or Tourette’s syndrome. Regularly monitor patients for the emergence or worsening of tics or Tourette’s syndrome. Discontinue treatment if clinically appropriate. (5.8)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥5% and at a rate at least twice placebo) in pediatric patients ages 6 to 17 years, and/or adults with ADHD were anorexia, anxiety, decreased appetite, decreased weight, diarrhea, dizziness, dry mouth, irritability, insomnia, nausea, upper abdominal pain, and vomiting. (6.1)
Most common adverse reactions (incidence ≥5% and at a rate at least twice placebo) in adults with BED were dry mouth, insomnia, decreased appetite, increased heart rate, constipation, feeling jittery, and anxiety. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Mallinckrodt at 1-800-778-7898 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Acidifying and Alkalinizing Agents: Agents that alter urinary pH can alter blood levels of amphetamine. Acidifying agents decrease amphetamine blood levels, while alkalinizing agents increase amphetamine blood levels. Adjust lisdexamfetamine dimesylate chewable tablets dosage accordingly. (2.6, 7.1)
USE IN SPECIFIC POPULATIONS
See 17 for Medication Guide.
Revised: 12/2024
Close -
-
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: ABUSE, MISUSE, AND ADDICTION
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Pretreatment Screening
2.2 General Administration Information
2.3 Dosage for Treatment of ADHD
2.4 Dosage for Treatment of Moderate to Severe BED in Adults
2.5 Dosage in Patients with Renal Impairment
2.6 Dosage Modifications Due to Drug Interactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Abuse, Misuse, and Addiction
5.2 Risks to Patients with Serious Cardiac Disease
5.3 Increased Blood Pressure and Heart Rate
5.4 Psychiatric Adverse Reactions
5.5 Long-Term Suppression of Growth in Pediatric Patients
5.6 Peripheral Vasculopathy, including Raynaud’s Phenomenon
5.7 Serotonin Syndrome
5.8 Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs Having Clinically Important Interactions with Amphetamines
7.2 Drugs Having No Clinically Important Interactions with Lisdexamfetamine Dimesylate Chewable Tablets
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Attention Deficit Hyperactivity Disorder (ADHD)
14.2 Binge Eating Disorder (BED)
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: ABUSE, MISUSE, AND ADDICTION
Lisdexamfetamine dimesylate chewable tablets have a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including lisdexamfetamine dimesylate chewable tablets, can result in overdose and death [see Overdosage (10)], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing lisdexamfetamine dimesylate chewable tablets, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug. Throughout lisdexamfetamine dimesylate chewable tablets treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction [see Warnings and Precautions (5.1), Drug Abuse and Dependence (9.2)].
Close
-
1 INDICATIONS AND USAGELisdexamfetamine dimesylate chewable tablets are indicated for the treatment of: Attention Deficit Hyperactivity Disorder (ADHD) in adults and pediatric patients 6 years and older [see Clinical ...
Lisdexamfetamine dimesylate chewable tablets are indicated for the treatment of:
- Attention Deficit Hyperactivity Disorder (ADHD) in adults and pediatric patients 6 years and older [see Clinical Studies (14.1)]
- Moderate to severe binge eating disorder (BED) in adults [see Clinical Studies (14.2)].
Limitations of Use:
- Pediatric patients with ADHD younger than 6 years of age experienced more long-term weight loss than patients 6 years and older [see Use in Specific Populations (8.4)].
- Lisdexamfetamine dimesylate chewable tablets are not indicated or recommended for weight loss. Use of other sympathomimetic drugs for weight loss has been associated with serious cardiovascular adverse events. The safety and effectiveness of lisdexamfetamine dimesylate chewable tablets for the treatment of obesity have not been established [see Warnings and Precautions (5.2)].
-
2 DOSAGE AND ADMINISTRATION2.1 Pretreatment Screening - Prior to treating patients with lisdexamfetamine dimesylate chewable tablets, assess: for the presence of cardiac disease (i.e., perform a careful ...
2.1 Pretreatment Screening
Prior to treating patients with lisdexamfetamine dimesylate chewable tablets, assess:
- for the presence of cardiac disease (i.e., perform a careful history, family history of sudden death or ventricular arrhythmia, and physical exam) [see Warnings and Precautions (5.2)].
-
the family history and clinically evaluate patients for motor or verbal tics or Tourette’s syndrome before initiating lisdexamfetamine dimesylate chewable tablets [see Warnings and Precautions (5.8)].
2.2 General Administration Information
Take lisdexamfetamine dimesylate chewable tablets orally in the morning with or without food; avoid afternoon doses because of the potential for insomnia. Lisdexamfetamine dimesylate chewable tablets may be administered in one of the following ways:
Information for lisdexamfetamine dimesylate chewable tablets:
- Lisdexamfetamine dimesylate chewable tablets must be chewed thoroughly before swallowing.
Vyvanse® Capsules can be substituted with lisdexamfetamine dimesylate chewable tablets on a unit per unit/mg per mg basis (for example, 30 mg capsules for 30 mg chewable tablet) [see Clinical Pharmacology (12.3)].
Do not take anything less than one chewable tablet per day. A single dose should not be divided.
2.3 Dosage for Treatment of ADHD
The recommended starting dosage in adults and pediatric patients 6 years and older is 30 mg once daily in the morning. Dosage may be adjusted in increments of 10 mg or 20 mg at approximately weekly intervals up to maximum recommended dosage of 70 mg once daily [see Clinical Studies (14.1)].
2.4 Dosage for Treatment of Moderate to Severe BED in Adults
The recommended starting dosage in adults is 30 mg once daily to be titrated in increments of 20 mg at approximately weekly intervals to achieve the recommended target dose of 50 mg to 70 mg once daily. The maximum recommended dosage is 70 mg once daily [see Clinical Studies (14.2)]. Discontinue lisdexamfetamine dimesylate chewable tablets if binge eating does not improve.
2.5 Dosage in Patients with Renal Impairment
In patients with severe renal impairment (GFR 15 to < 30 mL/min/1.73 m2), the maximum dosage should not exceed 50 mg once daily. In patients with end stage renal disease (ESRD, GFR < 15 mL/min/1.73 m2), the maximum recommended dosage is 30 mg once daily [see Use in Specific Populations (8.6)].
Close2.6 Dosage Modifications Due to Drug Interactions
Agents that alter urinary pH can impact urinary excretion and alter blood levels of amphetamine. Acidifying agents (e.g., ascorbic acid) decrease blood levels, while alkalinizing agents (e.g., sodium bicarbonate) increase blood levels. Adjust lisdexamfetamine dimesylate chewable tablets dosage accordingly [see Drug Interactions (7.1)].
-
3 DOSAGE FORMS AND STRENGTHSLisdexamfetamine dimesylate chewable tablets: Chewable tablets 10 mg: Round shape, white to off white tablet debossed “M” in a box on one side and 10 on the other - Chewable tablets 20 mg ...
Lisdexamfetamine dimesylate chewable tablets:
- Chewable tablets 10 mg: Round shape, white to off white tablet debossed “M” in a box on one side and 10 on the other
- Chewable tablets 20 mg: Hexagon shape, white to off white tablet debossed “M” in a box on one side and 20 on the other
- Chewable tablets 30 mg: Arc Triangle shape, white to off white tablet debossed “M” in a box on one side and 30 on the other
- Chewable tablets 40 mg: Capsule shape, white to off white tablet debossed “M” in a box on one side and 40 on the other
- Chewable tablets 50 mg: Arc Square shape, white to off white tablet debossed “M” in a box on one side and 50 on the other
- Chewable tablets 60 mg: Arc Diamond shape, white to off white tablet debossed “M” in a box on one side and 60 on the other
-
4 CONTRAINDICATIONSLisdexamfetamine dimesylate chewable tablets are contraindicated in patients with: Known hypersensitivity to amphetamine products or other ingredients of lisdexamfetamine dimesylate chewable ...
Lisdexamfetamine dimesylate chewable tablets are contraindicated in patients with:
- Known hypersensitivity to amphetamine products or other ingredients of lisdexamfetamine dimesylate chewable tablets. Anaphylactic reactions, Stevens-Johnson Syndrome, angioedema, and urticaria have been observed in postmarketing reports [see Adverse Reactions (6.2)].
- Patients taking monoamine oxidase inhibitors (MAOIs), or within 14 days of stopping MAOIs (including MAOIs such as linezolid or intravenous methylene blue), because of an increased risk of hypertensive crisis [see Warnings and Precautions (5.7) and Drug Interactions (7.1)].
-
5 WARNINGS AND PRECAUTIONS5.1 Abuse, Misuse, and Addiction - Lisdexamfetamine dimesylate chewable tablets have a high potential for abuse and misuse. The use of lisdexamfetamine dimesylate chewable tablets ...
5.1 Abuse, Misuse, and Addiction
Lisdexamfetamine dimesylate chewable tablets have a high potential for abuse and misuse. The use of lisdexamfetamine dimesylate chewable tablets exposes individuals to the risks of abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Lisdexamfetamine dimesylate chewable tablets can be diverted for non-medical use into illicit channels or distribution [see Drug Abuse and Dependence (9.2)]. Misuse and abuse of CNS stimulants, including lisdexamfetamine dimesylate chewable tablets, can result in overdose and death [see Overdosage (10)], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing lisdexamfetamine dimesylate chewable tablets, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks and proper disposal of any unused drug. Advise patients to store lisdexamfetamine dimesylate chewable tablets in a safe place, preferably locked, and instruct patients to not give lisdexamfetamine dimesylate chewable tablets to anyone else. Throughout lisdexamfetamine dimesylate chewable tablets treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
5.2 Risks to Patients with Serious Cardiac Disease
Sudden death has been reported in patients with structural cardiac abnormalities or other serious cardiac disease who were treated with CNS stimulants at the recommended ADHD dosage. Avoid lisdexamfetamine dimesylate chewable tablets use in patients with known structural cardiac abnormalities, cardiomyopathy, serious cardiac arrhythmia, coronary artery disease, or other serious cardiac disease.
5.3 Increased Blood Pressure and Heart Rate
CNS stimulants cause an increase in blood pressure (mean increase about 2 to 4 mm Hg) and heart rate (mean increase about 3 to 6 bpm). Some patients may have larger increases.
Monitor all lisdexamfetamine dimesylate chewable tablets-treated patients for potential tachycardia and hypertension.
5.4 Psychiatric Adverse Reactions
Exacerbation of Pre-existing Psychosis
CNS stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a pre-existing psychotic disorder.Induction of a Manic Episode in Patients with Bipolar Disorder
CNS stimulants may induce a manic or mixed episode. Prior to initiating lisdexamfetamine dimesylate chewable tablets treatment, screen patients for risk factors for developing a manic episode (e.g., comorbid or history of depressive symptoms or a family history of suicide, bipolar disorder, and depression).New Psychotic or Manic Symptoms
CNS stimulants, at the recommended dosage, may cause psychotic or manic symptoms (e.g., hallucinations, delusional thinking, or mania) in patients without a prior history of psychotic illness or mania. In a pooled analysis of multiple short-term, placebo-controlled studies of CNS stimulants, psychotic or manic symptoms occurred in approximately 0.1% of CNS stimulant-treated patients compared to 0% of placebo-treated patients. If such symptoms occur, consider discontinuing lisdexamfetamine dimesylate chewable tablets.5.5 Long-Term Suppression of Growth in Pediatric Patients
CNS stimulants have been associated with weight loss and slowing of growth rate in pediatric patients.
In a 4-week, placebo-controlled trial of lisdexamfetamine dimesylate in pediatric patients ages 6 to 12 years old with ADHD, there was a dose-related decrease in weight in the lisdexamfetamine dimesylate groups compared to weight gain in the placebo group. Additionally, in studies of another stimulant, there was slowing of the increase in height [see Adverse Reactions (6.1)].
Closely monitor growth (weight and height) in lisdexamfetamine dimesylate-treated pediatric patients. Patients who are not growing or gaining height or weight as expected may need to have their treatment interrupted. Lisdexamfetamine dimesylate is not approved for use in pediatric patients below 6 years of age [see Use in Specific Populations (8.4)].
5.6 Peripheral Vasculopathy, including Raynaud’s Phenomenon
CNS stimulants, including lisdexamfetamine dimesylate chewable tablets, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud’s phenomenon. Signs and symptoms are usually intermittent and mild; however, sequelae have included digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud’s phenomenon, were observed in postmarketing reports and at the therapeutic dosages of CNS stimulants in all age groups throughout the course of treatment. Signs and symptoms generally improved after dosage reduction or discontinuation of the CNS stimulant.
Careful observation for digital changes is necessary during lisdexamfetamine dimesylate chewable tablets treatment. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for lisdexamfetamine dimesylate chewable tablets-treated patients who develop signs or symptoms of peripheral vasculopathy.
5.7 Serotonin Syndrome
Serotonin syndrome, a potentially life-threatening reaction, may occur when amphetamines are used in combination with other drugs that affect the serotonergic neurotransmitter systems such as monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, and St. John’s Wort [see Drug Interactions (7.1)]. The co-administration with cytochrome P450 2D6 (CYP2D6) inhibitors may also increase the risk with increased exposure to the active metabolite of lisdexamfetamine dimesylate chewable tablets (dextroamphetamine). In these situations, consider an alternative non-serotonergic drug or an alternative drug that does not inhibit CYP2D6 [see Drug Interactions (7.1)].
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
Concomitant use of lisdexamfetamine dimesylate chewable tablets with MAOI drugs is contraindicated [see Contraindications (4)].
Discontinue treatment with lisdexamfetamine dimesylate chewable tablets and any concomitant serotonergic agents immediately if symptoms of serotonin syndrome occur, and initiate supportive symptomatic treatment. If concomitant use of lisdexamfetamine dimesylate chewable tablets with other serotonergic drugs or CYP2D6 inhibitors is clinically warranted, initiate lisdexamfetamine dimesylate chewable tablets with lower doses, monitor patients for the emergence of serotonin syndrome during drug initiation or titration, and inform patients of the increased risk for serotonin syndrome.
Close5.8 Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
CNS stimulants, including amphetamine, have been associated with the onset or exacerbation of motor and verbal tics. Worsening of Tourette’s syndrome has also been reported [see Adverse Reactions (6.2)].
Before initiating lisdexamfetamine dimesylate chewable tablets, assess the family history and clinically evaluate patients for tics or Tourette’s syndrome. Regularly monitor lisdexamfetamine dimesylate chewable tablets-treated patients for the emergence or worsening of tics or Tourette’s syndrome, and discontinue treatment if clinically appropriate.
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the labeling: Known hypersensitivity to amphetamine products or other ingredients of lisdexamfetamine ...
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Known hypersensitivity to amphetamine products or other ingredients of lisdexamfetamine dimesylate chewable tablets [see Contraindications (4)]
- Hypertensive Crisis When Used Concomitantly with Monoamine Oxidase Inhibitors [see Contraindications (4) and Drug Interactions (7.1)]
- Abuse, Misuse, and Addiction [see Boxed Warning, Warnings and Precautions (5.1), and Drug Abuse and Dependence (9.2, 9.3)]
- Risks to Patients with Serious Cardiac Disease [see Warnings and Precautions (5.2)]
- Increased Blood Pressure and Heart Rate [see Warnings and Precautions (5.3)]
- Psychiatric Adverse Reactions [see Warnings and Precautions (5.4)]
- Long-Term Suppression of Growth in Pediatric Patients [see Warnings and Precautions (5.5)]
- Peripheral Vasculopathy, including Raynaud’s Phenomenon [see Warnings and Precautions (5.6)]
- Serotonin Syndrome [see Warnings and Precautions (5.7)]
- Motor and Verbal Tics, and Worsening of Tourette’s Syndrome [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Attention Deficit Hyperactivity Disorder
The safety data in this section is based on data from the 4-week controlled parallel-group clinical studies of lisdexamfetamine dimesylate in pediatric and adult patients with ADHD [see Clinical Studies (14.1)].Adverse Reactions Associated with Discontinuation of Treatment in ADHD Clinical Trials
In the controlled trial in pediatric patients ages 6 to 12 years (Study 1), 8% (18/218) of lisdexamfetamine dimesylate-treated patients discontinued due to adverse reactions compared to 0% (0/72) of placebo-treated patients. The most frequently reported adverse reactions (1% or more and twice rate of placebo) were ECG voltage criteria for ventricular hypertrophy, tic, vomiting, psychomotor hyperactivity, insomnia, decreased appetite and rash [2 instances for each adverse reaction, i.e., 2/218 (1%)]. Less frequently reported adverse reactions (less than 1% or less than twice rate of placebo) included abdominal pain upper, dry mouth, weight decreased, dizziness, somnolence, logorrhea, chest pain, anger and hypertension.In the controlled trial in pediatric patients ages 13 to 17 years (Study 4), 3% (7/233) of lisdexamfetamine dimesylate-treated patients discontinued due to adverse reactions compared to 1% (1/77) of placebo-treated patients. The most frequently reported adverse reactions (1% or more and twice rate of placebo) were decreased appetite (2/233; 1%) and insomnia (2/233; 1%). Less frequently reported adverse reactions (less than 1% or less than twice rate of placebo) included irritability, dermatillomania, mood swings, and dyspnea.
In the controlled adult trial (Study 7), 6% (21/358) of lisdexamfetamine dimesylate-treated patients discontinued due to adverse reactions compared to 2% (1/62) of placebo-treated patients. The most frequently reported adverse reactions (1% or more and twice rate of placebo) were insomnia (8/358; 2%), tachycardia (3/358; 1%), irritability (2/358; 1%), hypertension (4/358; 1%), headache (2/358; 1%), anxiety (2/358; 1%), and dyspnea (3/358; 1%). Less frequently reported adverse reactions (less than 1% or less than twice rate of placebo) included palpitations, diarrhea, nausea, decreased appetite, dizziness, agitation, depression, paranoia and restlessness.
Adverse Reactions Occurring at an Incidence of ≥5% or More Among Lisdexamfetamine Dimesylate-Treated Patients with ADHD in Clinical Trials
The most common adverse reactions (incidence ≥5% and at a rate at least twice placebo) reported in pediatric patients ages 6 to 17 years, and/or adults were anorexia, anxiety, decreased appetite, decreased weight, diarrhea, dizziness, dry mouth, irritability, insomnia, nausea, upper abdominal pain, and vomiting.Adverse Reactions Occurring at an Incidence of 2% or More Among Lisdexamfetamine Dimesylate-Treated Patients with ADHD in Clinical Trials
Adverse reactions reported in the controlled trials in pediatric patients ages 6 to 12 years (Study 1), pediatric patients ages 13 to 17 years (Study 4), and adult patients (Study 7) treated with lisdexamfetamine dimesylate or placebo are presented in Tables 1, 2 and 3 below.Table 1 Adverse Reactions Reported by 2% or More of Pediatric Patients Ages 6 to 12 Years with ADHD Taking Lisdexamfetamine Dimesylate and Greater than or Equal to Twice the Incidence in Patients Taking Placebo in a 4-Week Clinical Trial (Study 1)
Lisdexamfetamine Dimesylate
(n=218)
Placebo
(n=72)
Decreased Appetite
39%
4%
Insomnia
22%
3%
Abdominal Pain Upper
12%
6%
Irritability
10%
0%
Vomiting
9%
4%
Weight Decreased
9%
1%
Nausea
6%
3%
Dry Mouth
5%
0%
Dizziness
5%
0%
Affect Lability
3%
0%
Rash
3%
0%
Pyrexia
2%
1%
Somnolence
2%
1%
Tic
2%
0%
Anorexia
2%
0%
Table 2 Adverse Reactions Reported by 2% or More of Pediatric Patients Ages 13 to 17 Years with ADHD Taking Lisdexamfetamine Dimesylate and Greater than or Equal to Twice the Incidence in Patients Taking Placebo in a 4-Week Clinical Trial (Study 4)
Lisdexamfetamine Dimesylate
(n=233)
Placebo
(n=77)
Decreased Appetite
34%
3%
Insomnia
13%
4%
Weight Decreased
9%
0%
Dry Mouth
4%
1%
Palpitations
2%
1%
Anorexia
2%
0%
Tremor
2%
0%
Table 3 Adverse Reactions Reported by 2% or More of Adult Patients with ADHD Taking Lisdexamfetamine Dimesylate and Greater than or Equal to Twice the Incidence in Patients Taking Placebo in a 4-Week Clinical Trial (Study 7)
Lisdexamfetamine Dimesylate
(n=358)
Placebo
(n=62)
Decreased Appetite
27%
2%
Insomnia
27%
8%
Dry Mouth
26%
3%
Diarrhea
7%
0%
Nausea
7%
0%
Anxiety
6%
0%
Anorexia
5%
0%
Feeling Jittery
4%
0%
Agitation
3%
0%
Increased Blood Pressure
3%
0%
Hyperhidrosis
3%
0%
Restlessness
3%
0%
Decreased Weight
3%
0%
Dyspnea
2%
0%
Increased Heart Rate
2%
0%
Tremor
2%
0%
Palpitations
2%
0%
In addition, in the adult population erectile dysfunction was observed in 2.6% of males on lisdexamfetamine dimesylate and 0% on placebo; decreased libido was observed in 1.4% of subjects on lisdexamfetamine dimesylate and 0% on placebo.
Weight Loss and Slowing Growth Rate in Pediatric Patients with ADHD
In a controlled trial of lisdexamfetamine dimesylate in pediatric patients ages 6 to 12 years (Study 1), mean weight loss from baseline after 4 weeks of therapy was -0.9, -1.9, and -2.5 pounds, respectively, for patients receiving 30 mg, 50 mg, and 70 mg of lisdexamfetamine dimesylate, compared to a 1 pound weight gain for patients receiving placebo. Higher doses were associated with greater weight loss with 4 weeks of treatment. Careful follow-up for weight in pediatric patients ages 6 to 12 years who received lisdexamfetamine dimesylate over 12 months suggests that consistently medicated pediatric patients (i.e., treatment for 7 days per week throughout the year) have a slowing in growth rate, measured by body weight as demonstrated by an age- and sex-normalized mean change from baseline in percentile, of -13.4 over 1 year (average percentiles at baseline and 12 months were 60.9 and 47.2, respectively). In a 4-week controlled trial of lisdexamfetamine dimesylate in pediatric patients ages 13 to 17 years, mean weight loss from baseline to endpoint was -2.7, -4.3, and -4.8 lbs., respectively, for patients receiving 30 mg, 50 mg, and 70 mg of lisdexamfetamine dimesylate, compared to a 2.0 pound weight gain for patients receiving placebo.Careful follow-up of weight and height in pediatric patients ages 7 to 10 years who were randomized to either methylphenidate or non-medication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and non-medication treated pediatric patients over 36 months (to the ages of 10 to 13 years), suggests that consistently medicated pediatric patients ages 7 to 13 years (i.e., treatment for 7 days per week throughout the year) have a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this period of development. In a controlled trial of amphetamine (d- to l-enantiomer ratio of 3:1) in pediatric patients ages 13 to 17 years, mean weight change from baseline within the initial 4 weeks of therapy was -1.1 pounds and -2.8 pounds, respectively, for patients receiving 10 mg and 20 mg of amphetamine. Higher doses were associated with greater weight loss within the initial 4 weeks of treatment [see Warnings and Precautions (5.5)].
Weight Loss in Adults with ADHD
In the controlled adult trial (Study 7), mean weight loss after 4 weeks of therapy was 2.8 pounds, 3.1 pounds, and 4.3 pounds, for patients receiving final doses of 30 mg, 50 mg, and 70 mg of lisdexamfetamine dimesylate, respectively, compared to a mean weight gain of 0.5 pounds for patients receiving placebo.Binge Eating Disorder
The safety data in this section is based on data from two 12-week parallel group, flexible-dose, placebo-controlled studies in adults with BED [see Clinical Studies 14.2]. Patients with cardiovascular risk factors other than obesity and smoking were excluded.Adverse Reactions Associated with Discontinuation of Treatment in BED Clinical Trials
In controlled trials of patients ages 18 to 55 years, 5.1% (19/373) of lisdexamfetamine dimesylate-treated patients discontinued due to adverse reactions compared to 2.4% (9/372) of placebo-treated patients. No single adverse reaction led to discontinuation in 1% or more of lisdexamfetamine dimesylate-treated patients. Less commonly reported adverse reactions (less than 1% or less than twice rate of placebo) included increased heart rate, headache, abdominal pain upper, dyspnea, rash, insomnia, irritability, feeling jittery and anxiety.Adverse Reactions Occurring at an Incidence of 5% or More and At Least Twice Placebo Among Lisdexamfetamine Dimesylate-Treated Patients with BED in Clinical Trials
The most common adverse reactions (incidence ≥5% and at a rate at least twice placebo) reported in adults were dry mouth, insomnia, decreased appetite, increased heart rate, constipation, feeling jittery, and anxiety.Adverse Reactions Occurring at an Incidence of 2% or More and At Least Twice Placebo Among Lisdexamfetamine Dimesylate-Treated Patients with BED in Clinical Trials
Adverse reactions reported in the pooled controlled trials in adult patients (Study 11 and 12) treated with lisdexamfetamine dimesylate or placebo are presented in Table 4 below.Table 4 Adverse Reactions Reported by 2% or More of Adult Patients with BED Taking Lisdexamfetamine Dimesylate and Greater than or Equal to Twice the Incidence in Patients Taking Placebo in 12-Week Clinical Trials (Study 11 and 12)
Lisdexamfetamine Dimesylate
(n=373)
Placebo
(n=372)
Dry Mouth
36%
7%
Insomnia1
20%
8%
Decreased Appetite
8%
2%
Increased Heart Rate2
7%
1%
Feeling Jittery
6%
1%
Constipation
6%
1%
Anxiety
5%
1%
Diarrhea
4%
2%
Decreased Weight
4%
0%
Hyperhidrosis
4%
0%
Vomiting
2%
1%
Gastroenteritis
2%
1%
Paresthesia
2%
1%
Pruritus
2%
1%
Upper Abdominal Pain
2%
0%
Energy Increased
2%
0%
Urinary Tract Infection
2%
0%
Nightmare
2%
0%
Restlessness
2%
0%
Oropharyngeal Pain
2%
0%
1 Includes all preferred terms containing the word “insomnia.”
2 Includes the preferred terms “heart rate increased” and “tachycardia.”
Close6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of lisdexamfetamine dimesylate chewable tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events are as follows: cardiomyopathy, mydriasis, diplopia, difficulties with visual accommodation, blurred vision, eosinophilic hepatitis, anaphylactic reaction, hypersensitivity, dyskinesia, dysgeusia, motor and verbal tics, bruxism, depression, dermatillomania, alopecia, aggression, Stevens-Johnson Syndrome, chest pain, angioedema, urticaria, seizures, libido changes, frequent or prolonged erections, constipation, rhabdomyolysis, and intestinal ischemia.
-
7 DRUG INTERACTIONS7.1 Drugs Having Clinically Important - Interactions with Amphetamines - Table 5 Drugs having clinically important interactions with amphetamines. MAO Inhibitors ...
7.1 Drugs Having Clinically Important Interactions with Amphetamines
Table 5 Drugs having clinically important interactions with amphetamines.
MAO Inhibitors (MAOI)
Clinical Impact
MAOI antidepressants slow amphetamine metabolism, increasing amphetamines effect on the release of norepinephrine and other monoamines from adrenergic nerve endings causing headaches and other signs of hypertensive crisis. Toxic neurological effects and malignant hyperpyrexia can occur, sometimes with fatal results.
Intervention
Do not administer lisdexamfetamine dimesylate chewable tablets during or within 14 days following the administration of MAOI [see Contraindications (4)].
Serotonergic Drugs
Clinical Impact
The concomitant use of lisdexamfetamine dimesylate chewable tablets and serotonergic drugs increases the risk of serotonin syndrome.
Intervention
Initiate with lower doses and monitor patients for signs and symptoms of serotonin syndrome, particularly during lisdexamfetamine dimesylate chewable tablets initiation or dosage increase. If serotonin syndrome occurs, discontinue lisdexamfetamine dimesylate chewable tablets and the concomitant serotonergic drug(s) [see Warnings and Precautions (5.7)].
CYP2D6 Inhibitors
Clinical Impact
The concomitant use of lisdexamfetamine dimesylate chewable tablets and CYP2D6 inhibitors may increase the exposure of dextroamphetamine, the active metabolite of lisdexamfetamine dimesylate chewable tablets compared to the use of the drug alone and increase the risk of serotonin syndrome.
Intervention
Initiate with lower doses and monitor patients for signs and symptoms of serotonin syndrome particularly during lisdexamfetamine dimesylate chewable tablets initiation and after a dosage increase. If serotonin syndrome occurs, discontinue lisdexamfetamine dimesylate chewable tablets and the CYP2D6 inhibitor [see Warnings and Precautions (5.7) and Overdosage (10)].
Alkalinizing Agents
Clinical Impact
Urinary alkalinizing agents can increase blood levels and potentiate the action of amphetamine.
Intervention
Co-administration of lisdexamfetamine dimesylate chewable tablets and urinary alkalinizing agents should be avoided.
Acidifying Agents
Clinical Impact
Urinary acidifying agents can lower blood levels and efficacy of amphetamines.
Intervention
Increase dose based on clinical response.
Tricyclic Antidepressants
Clinical Impact
May enhance the activity of tricyclic or sympathomimetic agents causing striking and sustained increases in the concentration of d-amphetamine in the brain; cardiovascular effects can be potentiated.
Intervention
Monitor frequently and adjust or use alternative therapy based on clinical response.
Close7.2 Drugs Having No Clinically Important Interactions with Lisdexamfetamine Dimesylate Chewable Tablets
From a pharmacokinetic perspective, no dose adjustment of lisdexamfetamine dimesylate chewable tablets is necessary when lisdexamfetamine dimesylate chewable tablets are co-administered with guanfacine, venlafaxine, or omeprazole. In addition, no dose adjustment of guanfacine or venlafaxine is needed when lisdexamfetamine dimesylate chewable tablets are co-administered [see Clinical Pharmacology (12.3)].
From a pharmacokinetic perspective, no dose adjustment for drugs that are substrates of CYP1A2 (e.g., theophylline, duloxetine, melatonin), CYP2D6 (e.g., atomoxetine, desipramine, venlafaxine), CYP2C19 (e.g., omeprazole, lansoprazole, clobazam), and CYP3A4 (e.g., midazolam, pimozide, simvastatin) is necessary when lisdexamfetamine dimesylate chewable tablets are co-administered [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The limited available data from published literature and postmarketing reports on use of lisdexamfetamine dimesylate chewable tablets in pregnant women are ...
8.1 Pregnancy
Risk Summary
The limited available data from published literature and postmarketing reports on use of lisdexamfetamine dimesylate chewable tablets in pregnant women are not sufficient to inform a drug-associated risk for major birth defects and miscarriage. Adverse pregnancy outcomes, including premature delivery and low birth weight, have been seen in infants born to mothers dependent on amphetamines [see Clinical Considerations]. In animal reproduction studies, lisdexamfetamine dimesylate (a prodrug of d-amphetamine) had no effects on embryo-fetal morphological development or survival when administered orally to pregnant rats and rabbits throughout the period of organogenesis. Pre- and postnatal studies were not conducted with lisdexamfetamine dimesylate. However, amphetamine (d- to l-ratio of 3:1) administration to pregnant rats during gestation and lactation caused a decrease in pup survival and a decrease in pup body weight that correlated with a delay in developmental landmarks at clinically relevant doses of amphetamine. In addition, adverse effects on reproductive performance were observed in pups whose mothers were treated with amphetamine. Long-term neurochemical and behavioral effects have also been reported in animal developmental studies using clinically relevant doses of amphetamine [see Data].The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Amphetamines, such as lisdexamfetamine dimesylate chewable tablets, cause vasoconstriction and thereby may decrease placental perfusion. In addition, amphetamines can stimulate uterine contractions increasing the risk of premature delivery. Infants born to amphetamine-dependent mothers have an increased risk of premature delivery and low birth weight.Monitor infants born to mothers taking amphetamines for symptoms of withdrawal such as feeding difficulties, irritability, agitation, and excessive drowsiness.
Data
Animal Data
Lisdexamfetamine dimesylate had no apparent effects on embryo-fetal morphological development or survival when administered orally to pregnant rats and rabbits throughout the period of organogenesis at doses of up to 40 and 120 mg/kg/day, respectively. These doses are approximately 5.5 and 33 times, respectively, the maximum recommended human dose (MRHD) of 70 mg/day given to adults, on a mg/m2 body surface area basis.A study was conducted with amphetamine (d- to l-enantiomer ratio of 3:1) in which pregnant rats received daily oral doses of 2, 6, and 10 mg/kg from gestation day 6 to lactation day 20. All doses caused hyperactivity and decreased weight gain in the dams. A decrease in pup survival was seen at all doses. A decrease in pup body weight was seen at 6 and 10 mg/kg which correlated with delays in developmental landmarks, such as preputial separation and vaginal opening. Increased pup locomotor activity was seen at 10 mg/kg on day 22 postpartum but not at 5 weeks postweaning. When pups were tested for reproductive performance at maturation, gestational weight gain, number of implantations, and number of delivered pups were decreased in the group whose mothers had been given 10 mg/kg.
A number of studies from the literature in rodents indicate that prenatal or early postnatal exposure to amphetamine (d- or d, l-) at doses similar to those used clinically can result in long-term neurochemical and behavioral alterations. Reported behavioral effects include learning and memory deficits, altered locomotor activity, and changes in sexual function.
8.2 Lactation
Risk Summary
Lisdexamfetamine is a prodrug of dextroamphetamine. Based on limited case reports in published literature, amphetamine (d- or d, l-) is present in human milk, at relative infant doses of 2% to 13.8% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 1.9 and 7.5. There are no reports of adverse effects on the breastfed infant. Long-term neurodevelopmental effects on infants from amphetamine exposure are unknown. It is possible that large dosages of dextroamphetamine might interfere with milk production, especially in women whose lactation is not well established. Because of the potential for serious adverse reactions in nursing infants, including serious cardiovascular reactions, blood pressure and heart rate increase, suppression of growth, and peripheral vasculopathy, advise patients that breastfeeding is not recommended during treatment with lisdexamfetamine dimesylate chewable tablets.8.4 Pediatric Use
ADHD
Safety and effectiveness of lisdexamfetamine dimesylate have been established in pediatric patients with ADHD ages 6 to 17 years [see Dosage and Administration (2.3), Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.1)].Safety and effectiveness of lisdexamfetamine dimesylate have not been established in pediatric patients below the age of 6 years.
Safety and efficacy of lisdexamfetamine dimesylate were evaluated in a double-blind, randomized, parallel-group, placebo-controlled, fixed-dose study in pediatric patients ages 4 to 5 years with ADHD, followed by a 1-year open-label extension study. In these studies, patients experienced elevated rates of adverse reactions, including weight loss, decreased BMI, decreased appetite, insomnia, infections (upper respiratory and nasopharyngitis), irritability, and affect lability.
With the same lisdexamfetamine dimesylate dose, mean steady state exposure of dextroamphetamine was approximately 44% higher in pediatric patients ages 4 to 5 years compared to the pediatric patients ages 6 to 11 years.
BED
Safety and effectiveness of lisdexamfetamine dimesylate have not been established in pediatric patients with BED less than 18 years of age.Growth Suppression
Growth should be monitored during treatment with stimulants, including lisdexamfetamine dimesylate, and pediatric patients who are not growing or gaining weight as expected may need to have their treatment interrupted [see Warnings and Precautions (5.5), Adverse Reactions (6.1)].Juvenile Animal Data
Studies conducted in juvenile rats and dogs at clinically relevant doses showed growth suppression that partially or fully reversed in dogs and female rats but not in male rats after a four-week drug-free recovery period.A study was conducted in which juvenile rats received oral doses of 4, 10, or 40 mg/kg/day of lisdexamfetamine dimesylate from day 7 to day 63 of age. These doses are approximately 0.3, 0.7, and 3 times the maximum recommended human daily dose of 70 mg on a mg/m2 basis for a child. Dose-related decreases in food consumption, bodyweight gain, and crown-rump length were seen; after a four-week drug-free recovery period, bodyweights and crown-rump lengths had significantly recovered in females but were still substantially reduced in males. Time to vaginal opening was delayed in females at the highest dose, but there were no drug effects on fertility when the animals were mated beginning on day 85 of age.
In a study in which juvenile dogs received lisdexamfetamine dimesylate for 6 months beginning at 10 weeks of age, decreased bodyweight gain was seen at all doses tested (2, 5, and 12 mg/kg/day, which are approximately 0.5, 1, and 3 times the maximum recommended human daily dose on a mg/m2 basis for a child). This effect partially or fully reversed during a four-week drug-free recovery period.
8.5 Geriatric Use
Clinical studies of lisdexamfetamine dimesylate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience and pharmacokinetic data [see Clinical Pharmacology (12.3)] have not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should start at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Close8.6 Renal Impairment
Due to reduced clearance in patients with severe renal impairment (GFR 15 to < 30 mL/min/1.73 m2), the maximum dose should not exceed 50 mg/day. The maximum recommended dose in ESRD (GFR < 15 mL/min/1.73 m2) patients is 30 mg/day [see Clinical Pharmacology (12.3)].
Lisdexamfetamine and d-amphetamine are not dialyzable.
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled - Substance - Lisdexamfetamine dimesylate chewable tablets contain lisdexamfetamine, a prodrug of amphetamine, a Schedule II controlled substance. 9.2 ...
9.1 Controlled Substance
Lisdexamfetamine dimesylate chewable tablets contain lisdexamfetamine, a prodrug of amphetamine, a Schedule II controlled substance.
9.2 Abuse
Lisdexamfetamine dimesylate chewable tablets have a high potential for abuse and misuse which can lead to the development of a substance use disorder, including addiction [see Warnings and Precautions (5.1)]. Lisdexamfetamine dimesylate chewable tablets can be diverted for non-medical use into illicit channels or distribution.
Abuse is the intentional non-therapeutic use of a drug, even once, to achieve a desired psychological or physiological effect. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a healthcare provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence.
Misuse and abuse of lisdexamfetamine, a prodrug of amphetamine, may cause increased heart rate, respiratory rate, or blood pressure; sweating; dilated pupils; hyperactivity; restlessness; insomnia; decreased appetite; loss of coordination; tremors; flushed skin; vomiting; and/or abdominal pain. Anxiety, psychosis, hostility, aggression, and suicidal or homicidal ideation have also been observed with CNS stimulants abuse and/or misuse. Misuse and abuse of CNS stimulants, including lisdexamfetamine dimesylate chewable tablets, can result in overdose and death [see Overdosage (10)], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Studies of Lisdexamfetamine Dimesylate in Drug Abusers
A randomized, double-blind, placebo-control, cross-over, abuse liability study in 38 patients with a history of drug abuse was conducted with single doses of 50, 100, or 150 mg of lisdexamfetamine dimesylate, 40 mg of immediate-release d-amphetamine sulphate (a controlled II substance), and 200 mg of diethylpropion hydrochloride (a controlled IV substance). Lisdexamfetamine dimesylate 100 mg produced significantly less “Drug Liking Effects” as measured by the Drug Rating Questionnaire-Subject score, compared to d-amphetamine 40 mg; and 150 mg of lisdexamfetamine dimesylate demonstrated similar “Drug-Liking Effects” compared to 40 mg of d-amphetamine and 200 mg of diethylpropion.Intravenous administration of 50 mg lisdexamfetamine dimesylate to individuals with a history of drug abuse produced positive subjective responses on scales measuring “Drug Liking”, “Euphoria”, “Amphetamine Effects”, and “Benzedrine Effects” that were greater than placebo but less than those produced by an equivalent dose (20 mg) of intravenous d-amphetamine.
Close9.3 Dependence
Physical Dependence
Lisdexamfetamine dimesylate chewable tablets may produce physical dependence. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Withdrawal signs and symptoms after abrupt discontinuation or dose reduction following prolonged use of CNS stimulants including lisdexamfetamine dimesylate chewable tablets include dysphoric mood; depression; fatigue; vivid, unpleasant dreams; insomnia or hypersomnia; increased appetite; and psychomotor retardation or agitation.Tolerance
Lisdexamfetamine dimesylate chewable tablets may produce tolerance. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose). -
10 OVERDOSAGEClinical Effects of Overdose - Overdose of CNS stimulants is characterized by the following sympathomimetic effects: Cardiovascular effects including tachyarrhythmias, and hypertension or ...
Clinical Effects of Overdose
Overdose of CNS stimulants is characterized by the following sympathomimetic effects:- Cardiovascular effects including tachyarrhythmias, and hypertension or hypotension. Vasospasm, myocardial infarction, or aortic dissection may precipitate sudden cardiac death. Takotsubo cardiomyopathy may develop.
- CNS effects including psychomotor agitation, confusion, and hallucinations. Serotonin syndrome, seizures, cerebral vascular accidents, and coma may occur.
- Life-threatening hyperthermia (temperatures greater than 104°F) and rhabdomyolysis may develop.
Overdose Management
Close
Consider the possibility of multiple drug ingestion. The pharmacokinetic profile of lisdexamfetamine dimesylate chewable tablets should be considered when treating patients with overdose. Lisdexamfetamine and
d-amphetamine are not dialyzable. Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations. -
11 DESCRIPTIONLisdexamfetamine dimesylate chewable tablets, a CNS stimulant, are for once-a-day oral administration. The chemical designation for lisdexamfetamine dimesylate is ...
Lisdexamfetamine dimesylate chewable tablets, a CNS stimulant, are for once-a-day oral administration. The chemical designation for lisdexamfetamine dimesylate is (2S)-2,6-diamino-N-[(1S)-1-methyl-2-phenylethyl] hexanamide dimethanesulfonate. The molecular formula is C15H25N3O•(CH4O3S)2, which corresponds to a molecular weight of 455.60. The chemical structure is:

Lisdexamfetamine dimesylate is a white to off-white powder that is soluble in water (792 mg/mL).
Information for lisdexamfetamine dimesylate chewable tablets:
Lisdexamfetamine dimesylate chewable tablets contain 10 mg, 20 mg, 30 mg, 40 mg, 50 mg, and 60 mg of lisdexamfetamine dimesylate (equivalent to 5.8 mg, 11.6 mg, 17.3 mg, 23.1 mg, 28.9 mg, and 34.7 mg of lisdexamfetamine).
Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, guar gum, magnesium stearate, mannitol, microcrystalline cellulose, sucralose, artificial strawberry flavor.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of - Action - Lisdexamfetamine is a prodrug of dextroamphetamine. Amphetamines are non-catecholamine sympathomimetic amines with CNS stimulant activity. The exact mode of ...
12.1 Mechanism of Action
Lisdexamfetamine is a prodrug of dextroamphetamine. Amphetamines are non-catecholamine sympathomimetic amines with CNS stimulant activity. The exact mode of therapeutic action in ADHD and BED is not known.
12.2 Pharmacodynamics
Amphetamines block the reuptake of norepinephrine and dopamine into the presynaptic neuron and increase the release of these monoamines into the extraneuronal space. The parent drug, lisdexamfetamine, does not bind to the sites responsible for the reuptake of norepinephrine and dopamine in vitro.
Close12.3 Pharmacokinetics
Pharmacokinetic studies after oral administration of lisdexamfetamine dimesylate have been conducted in healthy adult (capsule and chewable tablet formulations) and pediatric (6 to 12 years) patients with ADHD (capsule formulation). After single dose administration of lisdexamfetamine dimesylate, pharmacokinetics of dextroamphetamine was found to be linear between 30 mg and 70 mg in a pediatric study (6 to 12 years), and between 50 mg and 250 mg in an adult study. Dextroamphetamine pharmacokinetic parameters following administration of lisdexamfetamine dimesylate in adults exhibited low inter-subject (<25%) and intra-subject (<8%) variability. There is no accumulation of lisdexamfetamine and dextroamphetamine at steady state in healthy adults.
Absorption
Chewable Tablet Formulation
After a single dose administration of 60 mg lisdexamfetamine dimesylate chewable tablet in healthy subjects under fasted conditions, Tmax of lisdexamfetamine and dextroamphetamine was reached at approximately 1 hour and 4.4 hours post-dose, respectively. Compared to 60 mg Vyvanse® capsule, exposure (Cmax and AUC) to lisdexamfetamine was about 15% lower. The exposure (Cmax and AUCinf) of dextroamphetamine is similar between lisdexamfetamine dimesylate chewable tablet and Vyvanse® Capsules.Effect of Food on Tablet Formulation
Administration of 60 mg lisdexamfetamine dimesylate chewable tablet with food (a high-fat meal) decreases the exposure (Cmax and AUCinf) of dextroamphetamine by about 5% to 7%, and prolongs mean Tmax by approximately 1 hour (from 3.9 hours at fasted state to 4.9 hours).Elimination
Plasma concentrations of unconverted lisdexamfetamine are low and transient, generally becoming non-quantifiable by 8 hours after administration. The plasma elimination half-life of lisdexamfetamine typically averaged less than one hour in volunteers ages 6 years and older. The plasma elimination half-life of dextroamphetamine was approximately 8.6 to 9.5 hours in pediatric patients 6 to 12 years and 10 to 11.3 hours in healthy adults.Metabolism
Lisdexamfetamine is converted to dextroamphetamine and l-lysine primarily in blood due to the hydrolytic activity of red blood cells after oral administration of lisdexamfetamine dimesylate. In vitro data demonstrated that red blood cells have a high capacity for metabolism of lisdexamfetamine; substantial hydrolysis occurred even at low hematocrit levels (33% of normal). Lisdexamfetamine is not metabolized by cytochrome P450 enzymes.Excretion
Following oral administration of a 70 mg dose of radiolabeled lisdexamfetamine dimesylate to 6 healthy subjects, approximately 96% of the oral dose radioactivity was recovered in the urine and only 0.3% recovered in the feces over a period of 120 hours. Of the radioactivity recovered in the urine, 42% of the dose was related to amphetamine, 25% to hippuric acid, and 2% to intact lisdexamfetamine.Specific Populations
Exposures of dextroamphetamine in specific populations are summarized in Figure 1.Figure 1: Specific Populations*
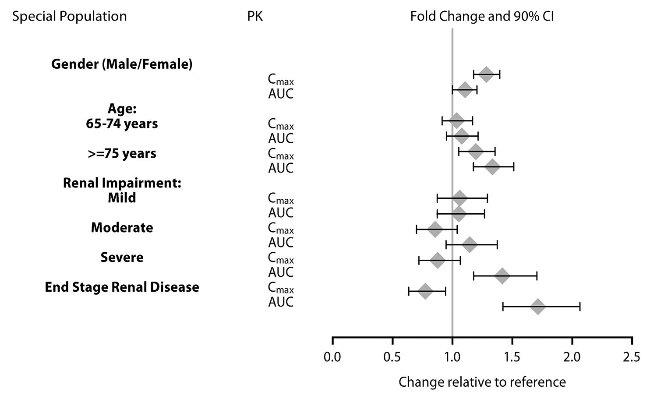
*Figure 1 shows the geometric mean ratios and the 90% confidence limits for Cmax and AUC of d-amphetamine. Comparison for gender uses males as the reference. Comparison for age uses 55 to 64 years as the reference.
Drug Interaction Studies
Effects of other drugs on the exposures of dextroamphetamine are summarized in Figure 2.Figure 2: Effect of Other Drugs on Lisdexamfetamine Dimesylate Chewable Tablets

The effects of lisdexamfetamine dimesylate chewable tablets on the exposures of other drugs are summarized in Figure 3.
Figure 3: Effect of Lisdexamfetamine Dimesylate Chewable Tablets on Other Drugs

-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility - Carcinogenesis - Carcinogenicity studies of lisdexamfetamine dimesylate have not been performed. No evidence of ...
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenesis
Carcinogenicity studies of lisdexamfetamine dimesylate have not been performed. No evidence of carcinogenicity was found in studies in which d-, l-amphetamine (enantiomer ratio of 1:1) was administered to mice and rats in the diet for 2 years at doses of up to 30 mg/kg/day in male mice, 19 mg/kg/day in female mice, and 5 mg/kg/day in male and female rats.Mutagenesis
Lisdexamfetamine dimesylate was not clastogenic in the mouse bone marrow micronucleus test in vivo and was negative when tested in the E. coli and S. typhimurium components of the Ames test and in the L5178Y/TK+/- mouse lymphoma assay in vitro.Impairment of Fertility
Amphetamine (d- to l-enantiomer ratio of 3:1) did not adversely affect fertility or early embryonic development in the rat at doses of up to 20 mg/kg/day.Close13.2 Animal Toxicology and/or Pharmacology
Acute administration of high doses of amphetamine (d- or d, l-) has been shown to produce long-lasting neurotoxic effects, including irreversible nerve fiber damage, in rodents. The significance of these findings to humans is unknown.
-
14 CLINICAL STUDIES14.1 Attention Deficit Hyperactivity Disorder (ADHD) Pediatric Patients Ages 6 to 12 Years with ADHD - A double-blind, randomized, placebo-controlled, parallel-group study (Study 1) was ...
14.1 Attention Deficit Hyperactivity Disorder (ADHD)
Pediatric Patients Ages 6 to 12 Years with ADHD
A double-blind, randomized, placebo-controlled, parallel-group study (Study 1) was conducted in pediatric patients ages 6 to 12 years (N=290) who met DSM-IV criteria for ADHD (either the combined type or the hyperactive-impulsive type). Patients were randomized to receive final doses of 30 mg, 50 mg, or 70 mg of lisdexamfetamine dimesylate or placebo once daily in the morning for a total of four weeks of treatment. All patients receiving lisdexamfetamine dimesylate were initiated on 30 mg for the first week of treatment. Patients assigned to the 50 mg and 70 mg dose groups were titrated by 20 mg per week until they achieved their assigned dose. The primary efficacy outcome was change in Total Score from baseline to endpoint in investigator ratings on the ADHD Rating Scale (ADHD-RS), an 18-item questionnaire with a score range of 0 to 54 points that measures the core symptoms of ADHD which includes both hyperactive/impulsive and inattentive subscales. Endpoint was defined as the last post-randomization treatment week (i.e., Weeks 1 through 4) for which a valid score was obtained. All lisdexamfetamine dimesylate dose groups were superior to placebo in the primary efficacy outcome. Mean effects at all doses were similar; however, the highest dose (70 mg/day) was numerically superior to both lower doses (Study 1 in Table 6). The effects were maintained throughout the day based on parent ratings (Conners’ Parent Rating Scale) in the morning (approximately 10 am), afternoon (approximately 2 pm), and early evening (approximately 6 pm).A double-blind, placebo-controlled, randomized, crossover design, analog classroom study (Study 2) was conducted in pediatric patients ages 6 to 12 years (N=52) who met DSM-IV criteria for ADHD (either the combined type or the hyperactive-impulsive type). Following a 3-week open-label dose optimization with Adderall XR®, patients were randomly assigned to continue their optimized dose of Adderall XR (10 mg, 20 mg, or 30 mg), lisdexamfetamine dimesylate (30 mg, 50 mg, or 70 mg), or placebo once daily in the morning for 1 week each treatment. Efficacy assessments were conducted at 1, 2, 3, 4.5, 6, 8, 10, and 12 hours post-dose using the Swanson, Kotkin, Agler, M.Flynn, and Pelham Deportment scores (SKAMP-DS), a 4-item subscale of the SKAMP with scores ranging from 0 to 24 points that measures deportment problems leading to classroom disruptions. A significant difference in patient behavior, based upon the average of investigator ratings on the SKAMP-DS across the 8 assessments were observed between patients when they received lisdexamfetamine dimesylate compared to patients when they received placebo (Study 2 in Table 6). The drug effect reached statistical significance from hours 2 to 12 post-dose, but was not significant at 1 hour.
A second double-blind, placebo-controlled, randomized, crossover design, analog classroom study (Study 3) was conducted in pediatric patients ages 6 to 12 years (N=129) who met DSM-IV criteria for ADHD (either the combined type or the hyperactive-impulsive type). Following a 4-week open-label dose optimization with lisdexamfetamine dimesylate (30 mg, 50 mg, 70 mg), patients were randomly assigned to continue their optimized dose of lisdexamfetamine dimesylate or placebo once daily in the morning for 1 week each treatment. A significant difference in patient behavior, based upon the average of investigator ratings on the SKAMP-Deportment scores across all 7 assessments conducted at 1.5, 2.5, 5.0, 7.5, 10.0, 12.0, and 13.0 hours post-dose, were observed between patients when they received lisdexamfetamine dimesylate compared to patients when they received placebo (Study 3 in Table 6, Figure 4).
Pediatric Patients Ages 13 to 17 Years with ADHD
A double-blind, randomized, placebo-controlled, parallel-group study (Study 4) was conducted in pediatric patients ages 13 to 17 years (N=314) who met DSM-IV criteria for ADHD. In this study, patients were randomized in a 1:1:1:1 ratio to a daily morning dose of lisdexamfetamine dimesylate (30 mg/day, 50 mg/day or 70 mg/day) or placebo for a total of four weeks of treatment. All patients receiving lisdexamfetamine dimesylate were initiated on 30 mg for the first week of treatment. Patients assigned to the 50 mg and 70 mg dose groups were titrated by 20 mg per week until they achieved their assigned dose. The primary efficacy outcome was change in Total Score from baseline to endpoint in investigator ratings on the ADHD Rating Scale (ADHD-RS). Endpoint was defined as the last post-randomization treatment week (i.e., Weeks 1 through 4) for which a valid score was obtained. All lisdexamfetamine dimesylate dose groups were superior to placebo in the primary efficacy outcome (Study 4 in Table 6).Pediatric Patients Ages 6 to 17 Years: Short-Term Treatment in ADHD
A double-blind, randomized, placebo- and active-controlled parallel-group, dose-optimization study (Study 5) was conducted in pediatric patients ages 6 to 17 years (n=336) who met DSM-IV criteria for ADHD. In this eight-week study, patients were randomized to a daily morning dose of lisdexamfetamine dimesylate (30, 50 or 70 mg/day), an active control, or placebo (1:1:1). The study consisted of a Screening and Washout Period (up to 42 days), a 7-week Double-Blind Evaluation Period (consisting of a 4-week Dose-Optimization Period followed by a 3-week Dose-Maintenance Period), and a 1-week Washout and Follow-up Period. During the Dose-Optimization Period, subjects were titrated until an optimal dose, based on tolerability and investigator’s judgment, was reached. Lisdexamfetamine dimesylate showed significantly greater efficacy than placebo. The placebo-adjusted mean reduction from baseline in the ADHD-RS-IV total score was 18.6. Subjects on lisdexamfetamine dimesylate also showed greater improvement on the Clinical Global Impression-Improvement (CGI-I) rating scale compared to subjects on placebo (Study 5 in Table 6).Pediatric Patients Ages 6 to 17 Years: Maintenance Treatment in ADHD
Maintenance of Efficacy Study (Study 6) - A double-blind, placebo-controlled, randomized withdrawal study was conducted in pediatric patients ages 6 to 17 years (N=276) who met the diagnosis of ADHD (DSM-IV criteria). A total of 276 patients were enrolled into the study, 236 patients participated in Study 5 and 40 subjects directly enrolled. Subjects were treated with open-label lisdexamfetamine dimesylate for at least 26 weeks prior to being assessed for entry into the randomized withdrawal period. Eligible patients had to demonstrate treatment response as defined by CGI-S <3 and Total Score on the ADHD-RS ≤22. Patients that maintained treatment response for 2 weeks at the end of the open label treatment period were eligible to be randomized to ongoing treatment with the same dose of lisdexamfetamine dimesylate (N=78) or switched to placebo (N=79) during the double-blind phase. Patients were observed for relapse (treatment failure) during the 6 week double-blind phase. A significantly lower proportion of treatment failures occurred among lisdexamfetamine dimesylate subjects (15.8%) compared to placebo (67.5%) at endpoint of the randomized withdrawal period. The endpoint measurement was defined as the last post-randomization treatment week at which a valid ADHD-RS Total Score and CGI-S were observed. Treatment failure was defined as a ≥50% increase (worsening) in the ADHD-RS Total Score and a ≥2-point increase in the CGI-S score compared to scores at entry into the double-blind randomized withdrawal phase. Subjects who withdrew from the randomized withdrawal period and who did not provide efficacy data at their last on-treatment visit were classified as treatment failures (Study 6, Figure 5).Adults: Short-Term Treatment in ADHD
A double-blind, randomized, placebo-controlled, parallel-group study (Study 7) was conducted in adults ages 18 to 55 (N=420) who met DSM-IV criteria for ADHD. In this study, patients were randomized to receive final doses of 30 mg, 50 mg, or 70 mg of lisdexamfetamine dimesylate or placebo for a total of four weeks of treatment. All patients receiving lisdexamfetamine dimesylate were initiated on 30 mg for the first week of treatment. Patients assigned to the 50 mg and 70 mg dose groups were titrated by 20 mg per week until they achieved their assigned dose. The primary efficacy outcome was change in Total Score from baseline to endpoint in investigator ratings on the ADHD Rating Scale (ADHD-RS). Endpoint was defined as the last post-randomization treatment week (i.e., Weeks 1 through 4) for which a valid score was obtained. All lisdexamfetamine dimesylate dose groups were superior to placebo in the primary efficacy outcome (Study 7 in Table 6).The second study was a multi-center, randomized, double-blind, placebo-controlled, cross-over, modified analog classroom study (Study 8) of lisdexamfetamine dimesylate to simulate a workplace environment in 142 adults ages 18 to 55 who met DSM-IV-TR criteria for ADHD. There was a 4-week open-label, dose optimization phase with lisdexamfetamine dimesylate (30 mg/day, 50 mg/day, or 70 mg/day in the morning). Patients were then randomized to one of two treatment sequences: 1) lisdexamfetamine dimesylate (optimized dose) followed by placebo, each for one week, or 2) placebo followed by lisdexamfetamine dimesylate, each for one week. Efficacy assessments occurred at the end of each week, using the Permanent Product Measure of Performance (PERMP), a skill-adjusted math test that measures attention in ADHD. PERMP total score results from the sum of the number of math problems attempted plus the number of math problems answered correctly. Lisdexamfetamine dimesylate treatment, compared to placebo, resulted in a statistically significant improvement in attention across all post-dose time-points, as measured by average PERMP total scores over the course of one assessment day, as well as at each time-point measured. The PERMP assessments were administered at pre-dose (-0.5 hours) and at 2, 4, 8, 10, 12, and 14 hours post-dose (Study 8 in Table 6, Figure 6).
Adults: Maintenance Treatment in ADHD
A double-blind, placebo-controlled, randomized withdrawal design study (Study 9) was conducted in adults ages 18 to 55 (N=123) who had a documented diagnosis of ADHD or met DSM-IV criteria for ADHD. At study entry, patients must have had documentation of treatment with lisdexamfetamine dimesylate for a minimum of 6 months and had to demonstrate treatment response as defined by Clinical Global Impression Severity (CGI-S) ≤3 and Total Score on the ADHD-RS <22. ADHD-RS Total Score is a measure of core symptoms of ADHD. The CGI-S score assesses the clinician’s impression of the patient’s current illness state and ranges from 1 (not at all ill) to 7 (extremely ill). Patients that maintained treatment response at Week 3 of the open label treatment phase (N=116) were eligible to be randomized to ongoing treatment with the same dose of lisdexamfetamine dimesylate (N=56) or switched to placebo (N=60) during the double-blind phase. Patients were observed for relapse (treatment failure) during the 6-week double-blind phase. The efficacy endpoint was the proportion of patients with treatment failure during the double-blind phase. Treatment failure was defined as a ≥50% increase (worsening) in the ADHD-RS Total Score and ≥2-point increase in the CGI-S score compared to scores at entry into the double-blind phase. Maintenance of efficacy for patients treated with lisdexamfetamine dimesylate was demonstrated by the significantly lower proportion of patients with treatment failure (9%) compared to patients receiving placebo (75%) at endpoint during the double-blind phase (Study 9, Figure 7).Table 6 Summary of Primary Efficacy Results from Short-Term Studies of Lisdexamfetamine Dimesylate in Pediatric Patients (Ages 6 to 17) and Adults with ADHD
Study Number
(Age range)Primary Endpoint
Treatment Group
Mean Baseline Score (SD)
LS Mean Change from Baseline (SE)
Placebo-subtracted Difference*
(95% CI)Study 1
(6 - 12 years)ADHD-RS-IV
Lisdexamfetamine Dimesylate (30 mg/day)†
Lisdexamfetamine Dimesylate (50 mg/day)†
Lisdexamfetamine Dimesylate (70 mg/day)†
Placebo
43.2 (6.7)
43.3 (6.7)
45.1 (6.8)
42.4 (7.1)
-21.8 (1.6)
-23.4 (1.6)
-26.7 (1.5)
-6.2 (1.6)
-15.5 (-19.9, -11.2)
-17.2 (-21.5, -12.9)
-20.5 (-24.8, -16.2)
--
Study 2
(6 - 12 years)Average SKAMP-DS
Lisdexamfetamine Dimesylate (30, 50 or 70 mg/day)†
Placebo
--‡
--‡
0.8 (0.1)§
1.7 (0.1)§
-0.9 (-1.1, -0.7)
--
Study 3
(6 - 12 years)Average SKAMP-DS
Lisdexamfetamine Dimesylate (30, 50 or 70 mg/day)†
Placebo
0.9 (1.0)¶
0.7 (0.9)¶
0.7 (0.1)§
1.4 (0.1)§
-0.7 (-0.9, -0.6)
--
Study 4
(13 - 17 years)ADHD-RS-IV
Lisdexamfetamine Dimesylate (30 mg/day)†
Lisdexamfetamine Dimesylate (50 mg/day)†
Lisdexamfetamine Dimesylate (70 mg/day)†
Placebo
38.3 (6.7)
37.3 (6.3)
37.0 (7.3)
38.5 (7.1)
-18.3 (1.2)
-21.1 (1.3)
-20.7 (1.3)
-12.8 (1.2)
-5.5 (-9.0, -2.0)
-8.3 (-11.8, -4.8)
-7.9 (-11.4, -4.5)
--
Study 5
(6 - 17 years)ADHD-RS-IV
Lisdexamfetamine Dimesylate (30, 50 or 70 mg/day)†
Placebo
40.7 (7.3)
41.0 (7.1)
-24.3 (1.2)
-5.7 (1.1)
-18.6 (-21.5, -15.7)
--
Study 7
(18 - 55 years)ADHD-RS-IV
Lisdexamfetamine Dimesylate (30 mg/day)†
Lisdexamfetamine Dimesylate (50 mg/day)†
Lisdexamfetamine Dimesylate (70 mg/day)†
Placebo
40.5 (6.2)
40.8 (7.3)
41.0 (6.0)
39.4 (6.4)
-16.2 (1.1)
-17.4 (1.0)
-18.6 (1.0)
-8.2 (1.4)
-8.0 (-11.5, -4.6)
-9.2 (-12.6, -5.7)
-10.4 (-13.9, -6.9)
--
Study 8
(18 - 55 years)Average PERMP
Lisdexamfetamine Dimesylate (30, 50 or 70 mg/day)†
Placebo
260.1 (86.2)¶
261.4 (75.0)¶
312.9 (8.6)§
289.5 (8.6)§
23.4 (15.6, 31.2)
--
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval.
* Difference (drug minus placebo) in least-squares mean change from baseline.
† Doses statistically significantly superior to placebo.
‡ Pre-dose SKAMP-DS was not collected.
§ LS Mean for SKAMP-DS (Study 2 and 3) or PERMP (Study 8) is post-dose average score over all sessions of the treatment day, rather than change from baseline.
¶ Pre-dose SKAMP-DS (Study 3) or PERMP (Study 8) total score, averaged over both periods.Figure 4: LS Mean SKAMP Deportment Subscale Score by Treatment and Time-Point for Pediatric Patients Ages 6 to 12 with ADHD after 1 Week of Double-Blind Treatment (Study 3)

Higher score on the SKAMP-Deportment scale indicates more severe symptoms
Figure 5: Kaplan-Meier Estimated Proportion of Patients with Treatment Failure for Pediatric Patients Ages 6 to 17 (Study 6)

Figure 6: LS Mean (SE) PERMP Total Score by Treatment and Time-Point for Adults Ages 18 to 55 with ADHD after 1 Week of Double-Blind Treatment (Study 8)

Higher score on the PERMP scale indicates less severe symptoms.Figure 7: Kaplan-Meier Estimated Proportion of Subjects with Relapse in Adults with ADHD (Study 9)
 Close
Close14.2 Binge Eating Disorder (BED)
A phase 2 study evaluated the efficacy of lisdexamfetamine dimesylate 30, 50 and 70 mg/day compared to placebo in reducing the number of binge days/week in adults with at least moderate to severe BED. This randomized, double-blind, parallel-group, placebo-controlled, forced-dose titration study (Study 10) consisted of an 11-week double-blind treatment period (3 weeks of forced-dose titration followed by 8 weeks of dose maintenance). Lisdexamfetamine dimesylate 30 mg/day were not statistically different from placebo on the primary endpoint. The 50 and 70 mg/day doses were statistically superior to placebo on the primary endpoint.
The efficacy of lisdexamfetamine dimesylate in the treatment of BED was demonstrated in two 12-week randomized, double-blind, multi-center, parallel-group, placebo-controlled, dose-optimization studies (Study 11 and Study 12) in adults aged 18 to 55 years (Study 11: N=374, Study 12: N=350) with moderate to severe BED. A diagnosis of BED was confirmed using DSM-IV criteria for BED. Severity of BED was determined based on having at least 3 binge days per week for 2 weeks prior to the baseline visit and on having a Clinical Global Impression Severity (CGI-S) score of ≥4 at the baseline visit. For both studies, a binge day was defined as a day with at least 1 binge episode, as determined from the subject’s daily binge diary.
Both 12-week studies consisted of a 4-week dose-optimization period and an 8-week dose-maintenance period. During dose-optimization, subjects assigned to lisdexamfetamine dimesylate began treatment at the titration dose of 30 mg/day and, after 1 week of treatment, were subsequently titrated to 50 mg/day. Additional increases to 70 mg/day were made as tolerated and clinically indicated. Following the dose-optimization period, subjects continued on their optimized dose for the duration of the dose-maintenance period.
The primary efficacy outcome for the two studies was defined as the change from baseline at Week 12 in the number of binge days per week. Baseline is defined as the weekly average of the number of binge days per week for the 14 days prior to the baseline visit. Subjects from both studies on lisdexamfetamine dimesylate had a statistically significantly greater reduction from baseline in mean number of binge days per week at Week 12. In addition, subjects on lisdexamfetamine dimesylate showed greater improvement as compared to placebo across key secondary outcomes with higher proportion of subjects rated improved on the CGI-I rating scale, higher proportion of subjects with 4-week binge cessation, and greater reduction in the Yale-Brown Obsessive Compulsive Scale Modified for Binge Eating (Y-BOCS-BE) total score.
Table 7 Summary of Primary Efficacy Results in BED
Study Number
Treatment Group
Primary Efficacy Measure: Binge Days per Week at Week 12
Mean Baseline Score (SD)
LS Mean Change from Baseline (SE)
Placebo-subtracted Difference*
(95% CI)Study 11
Lisdexamfetamine Dimesylate (30, 50 or 70 mg/day)†
Placebo
4.79 (1.27)
4.60 (1.21)
-3.87 (0.12)
-2.51 (0.13)
-1.35 (-1.70, -1.01)
--
Study 12
Lisdexamfetamine Dimesylate (30, 50 or 70 mg/day)†
Placebo
4.66 (1.27)
4.82 (1.42)
-3.92 (0.14)
-2.26 (0.14)
-1.66 (-2.04, -1.28)
--
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval.
* Difference (drug minus placebo) in least-squares mean change from baseline.
† Doses statistically significantly superior to placebo.A double-blind, placebo controlled, randomized withdrawal design study (Study 13) was conducted to evaluate maintenance of efficacy based on time to relapse between lisdexamfetamine dimesylate and placebo in adults aged 18 to 55 (N=267) with moderate to severe BED. In this longer-term study patients who had responded to lisdexamfetamine dimesylate in the preceding 12-week open-label treatment phase were randomized to continuation of lisdexamfetamine dimesylate or placebo for up to 26 weeks of observation for relapse. Response in the open-label phase was defined as 1 or fewer binge days each week for four consecutive weeks prior to the last visit at the end of the 12-week open-label phase and a CGI-S score of 2 or less at the same visit. Relapse during the double-blind phase was defined as having 2 or more binge days each week for two consecutive weeks (14 days) prior to any visit and having an increase in CGI-S score of 2 or more points compared to the randomized-withdrawal baseline. Maintenance of efficacy for patients who had an initial response during the open-label period and then continued on lisdexamfetamine dimesylate during the 26-week double-blind randomized-withdrawal phase was demonstrated with lisdexamfetamine dimesylate being superior over placebo as measured by time to relapse.
Figure 8: Kaplan-Meier Estimated Proportion of Subjects with Relapse in Adults with BED (Study 13)

Examination of population subgroups based on age (there were no patients over 65), gender, and race did not reveal any clear evidence of differential responsiveness in the treatment of BED.
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Lisdexamfetamine Dimesylate Chewable Tablets: Lisdexamfetamine Dimesylate Chewable Tablets 10 mg: Round shape, white to off white tablet debossed “M” in a box on one ...
16.1 How Supplied
Lisdexamfetamine Dimesylate Chewable Tablets:
- Lisdexamfetamine Dimesylate Chewable Tablets 10 mg: Round shape, white to off white tablet debossed “M” in a box on one side and 10 on the other
Bottles of 100.................................................. NDC 0406-5124-01 - Lisdexamfetamine Dimesylate Chewable Tablets 20 mg: Hexagon shape, white to off white tablet debossed “M” in a box on one side and 20 on the other
Bottles of 100.................................................. NDC 0406-5125-01 - Lisdexamfetamine Dimesylate Chewable Tablets 30 mg: Arc Triangle shape, white to off white tablet debossed “M” in a box on one side and 30 on the other
Bottles of 100.................................................. NDC 0406-5126-01
- Lisdexamfetamine Dimesylate Chewable Tablets 40 mg: Capsule shape, white to off white tablet debossed “M” in a box on one side and 40 on the other
Bottles of 100.................................................. NDC 0406-5127-01
- Lisdexamfetamine Dimesylate Chewable Tablets 50 mg: Arc Square shape, white to off white tablet debossed “M” in a box on one side and 50 on the other
Bottles of 100.................................................. NDC 0406-5128-01
- Lisdexamfetamine Dimesylate Chewable Tablets 60 mg: Arc Diamond shape, white to off white tablet debossed “M” in a box on one side and 60 on the other
Bottles of 100.................................................. NDC 0406-5129-01
Close16.2 Storage and Handling
Dispense in a tight, light-resistant container as defined in the USP.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
- Lisdexamfetamine Dimesylate Chewable Tablets 10 mg: Round shape, white to off white tablet debossed “M” in a box on one side and 10 on the other
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Abuse, Misuse, and Addiction - Educate patients and their families about the risks of abuse, misuse, and ...
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Abuse, Misuse, and Addiction
Educate patients and their families about the risks of abuse, misuse, and addiction of lisdexamfetamine dimesylate chewable tablets, which can lead to overdose and death, and proper disposal of any unused drug [see Warnings and Precautions (5.1), Drug Abuse and Dependence (9.2), Overdosage (10)]. Advise patients to store lisdexamfetamine dimesylate chewable tablets in a safe place, preferably locked, and instruct patients to not give lisdexamfetamine dimesylate chewable tablets to anyone else.Risks to Patients with Serious Cardiac Disease
Advise patients that there are potential risks to patients with serious cardiac disease, including sudden death, with lisdexamfetamine dimesylate chewable tablets use. Instruct patients to contact a healthcare provider immediately if they develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease [see Warnings and Precautions (5.2)].Increased Blood Pressure and Heart Rate
Instruct patients that lisdexamfetamine dimesylate chewable tablets can cause elevations of their blood pressure and pulse rate and they should be monitored for such effects.Psychiatric Adverse Reactions
Advise patients that lisdexamfetamine dimesylate chewable tablets at recommended doses may cause psychotic or manic symptoms even in patients without prior history of psychotic symptoms or mania [see Warnings and Precautions (5.4)].Long-Term Suppression of Growth in Pediatric Patients
Advise patients that lisdexamfetamine dimesylate chewable tablets may cause slowing of growth including weight loss [see Warnings and Precautions (5.5)].Circulation Problems in Fingers and Toes [Peripheral Vasculopathy, Including Raynaud’s Phenomenon]
Instruct patients beginning treatment with lisdexamfetamine dimesylate chewable tablets about the risk of peripheral vasculopathy, including Raynaud’s phenomenon, and associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change from pale, to blue, to red. Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes. Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking lisdexamfetamine dimesylate chewable tablets. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients [see Warnings and Precautions (5.6)].Serotonin Syndrome
Caution patients about the risk of serotonin syndrome with concomitant use of lisdexamfetamine dimesylate chewable tablets and other serotonergic drugs including SSRIs, SNRIs, triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, St. John’s Wort, and with drugs that impair metabolism of serotonin (in particular MAOIs, both those intended to treat psychiatric disorders and also others such as linezolid [see Contraindications (4), Warnings and Precautions (5.7) and Drug Interactions (7.1)]. Advise patients to contact their healthcare provider or report to the emergency room if they experience signs or symptoms of serotonin syndrome.Concomitant Medications
Advise patients to notify their physicians if they are taking, or plan to take, any prescription or over-the-counter drugs because there is a potential for interactions [see Drug Interactions (7.1)].Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
Advise patients that motor and verbal tics and worsening of Tourette’s syndrome may occur during treatment with lisdexamfetamine dimesylate chewable tablets. Instruct patients to notify their healthcare provider if emergence of new tics or worsening of tics or Tourette’s syndrome occurs [see Warnings and Precautions (5.8)].Pregnancy
Advise patients of the potential fetal effects from the use of lisdexamfetamine dimesylate chewable tablets during pregnancy. Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with lisdexamfetamine dimesylate chewable tablets [see Use in Specific Populations (8.1)].Lactation
Advise women not to breastfeed if they are taking lisdexamfetamine dimesylate chewable tablets [see Use in Specific Populations (8.2)].Administration Instructions
- Chewable Tablets: Advise patients that chewable tablets must be chewed thoroughly before swallowing [see Dosage and Administration (2.2)].
Manufactured for:
SpecGx LLC
Webster Groves, MO 63119 USAFor more information call 1-800-778-7898
All Product/Brand names are the trademarks of their respective owners.
Mallinckrodt, the “M” brand mark and the Mallinckrodt Pharmaceuticals logo are trademarks of a Mallinckrodt company.
© 2024 Mallinckrodt.
Mallinckrodt™
PharmaceuticalsRev 08/2024
Close -
MEDICATION GUIDEAn electronic copy of this medication guide can be obtained from www.mallinckrodt.com/Medguide/MG20L05.pdf or by calling 1-800-778-7898 for alternate delivery options. MEDICATION GUIDE ...
An electronic copy of this medication guide can be obtained from www.mallinckrodt.com/Medguide/MG20L05.pdf or by calling 1-800-778-7898 for alternate delivery options.
MEDICATION GUIDE
Lisdexamfetamine Dimesylate
(lis dex″ am fet′ a meen dye mes′ i late)
Chewable Tablets, CII
What is the most important information I should know about lisdexamfetamine dimesylate chewable tablets?
Lisdexamfetamine dimesylate chewable tablets may cause serious side effects, including:
- Abuse, misuse, and addiction. Lisdexamfetamine dimesylate chewable tablets have a high chance for abuse and misuse and may lead to substance use problems, including addiction. Misuse and abuse of lisdexamfetamine dimesylate chewable tablets, other amphetamine containing medicines, and methylphenidate containing medicines, can lead to overdose and death. The risk of overdose and death is increased with higher doses of lisdexamfetamine dimesylate chewable tablets or when they are used in ways that are not approved, such as snorting or injection.
- Your healthcare provider should check you or your child’s risk for abuse, misuse, and addiction before starting treatment with lisdexamfetamine dimesylate chewable tablets and will monitor you or your child during treatment.
- Lisdexamfetamine dimesylate chewable tablets may lead to physical dependence after prolonged use, even if taken as directed by your healthcare provider.
- Do not give lisdexamfetamine dimesylate chewable tablets to anyone else. See “What are lisdexamfetamine dimesylate chewable tablets?” for more information.
- Keep lisdexamfetamine dimesylate chewable tablets in a safe place and properly dispose of any unused medicine. See “How should I store lisdexamfetamine dimesylate chewable tablets?” for more information.
- Tell your healthcare provider if you or your child have ever abused or been dependent on alcohol, prescription medicines, or street drugs.
- Risks for people with serious heart disease. Sudden death has happened in people who have heart defects or other serious heart disease.
Your healthcare provider should check you or your child carefully for heart problems before starting treatment with lisdexamfetamine dimesylate chewable tablets. Tell your healthcare provider if you or your child have any heart problems, heart disease, or heart defects.
Call your healthcare provider or go to the nearest hospital emergency room right away if you or your child have any signs of heart problems such as chest pain, shortness of breath, or fainting during treatment with lisdexamfetamine dimesylate chewable tablets.
- Increased blood pressure and heart rate.
Your healthcare provider should check you or your child’s blood pressure and heart rate regularly during treatment with lisdexamfetamine dimesylate chewable tablets.
- Mental (psychiatric) problems, including:
- new or worse behavior and thought problems
- new or worse bipolar illness
- new psychotic symptoms (such as hearing voices, or seeing or believing things that are not real) or new manic symptoms
Tell your healthcare provider about any mental problems you or your child have or about a family history of suicide, bipolar illness, or depression.
Call your healthcare provider right away if you or your child have any new or worsening mental symptoms or problems during treatment with lisdexamfetamine dimesylate chewable tablets, especially hearing voices, seeing or believing things that are not real, or new manic symptoms.
What are lisdexamfetamine dimesylate chewable tablets?
Lisdexamfetamine dimesylate chewable tablets are a central nervous system (CNS) stimulant prescription medicine used for the treatment of:
- Attention Deficit Hyperactivity Disorder (ADHD) in adults and children 6 years of age and older. Lisdexamfetamine dimesylate chewable tablets may help increase attention and decrease impulsiveness and hyperactivity in people with ADHD.
- Moderate to severe binge eating disorder (BED) in adults. Lisdexamfetamine dimesylate chewable tablets may help reduce the number of binge eating days in people with BED.
Lisdexamfetamine dimesylate chewable tablets are not for use in children under 6 years of age with ADHD.
Lisdexamfetamine dimesylate chewable tablets are not for weight loss. It is not known if lisdexamfetamine dimesylate chewable tablets are safe and effective for the treatment of obesity.
It is not known if lisdexamfetamine dimesylate chewable tablets are safe and effective for use in children with BED.
Lisdexamfetamine dimesylate chewable tablets are a federally controlled substance (CII) because it contains lisdexamfetamine dimesylate that can be a target for people who abuse prescription medicines or street drugs. Keep lisdexamfetamine dimesylate chewable tablets in a safe place to protect it from theft. Never give your lisdexamfetamine dimesylate chewable tablets to anyone else because it may cause death or harm them. Selling or giving away lisdexamfetamine dimesylate chewable tablets may harm others and is against the law.
Do not take lisdexamfetamine dimesylate chewable tablets if you or your child are:
- allergic to amphetamine products or any of the ingredients in lisdexamfetamine dimesylate chewable tablets. See the end of this Medication Guide for a complete list of ingredients in lisdexamfetamine dimesylate chewable tablets.
- taking, or have stopped taking in the last 14 days, a medicine called a Monoamine Oxidase Inhibitor (MAOI).
- being treated with the antibiotic linezolid or intravenous methylene blue.
Before taking lisdexamfetamine dimesylate chewable tablets, tell your healthcare provider about all medical conditions, including if you or your child:
- have heart problems, heart disease, heart defects, or high blood pressure
- have mental problems including psychosis, mania, bipolar illness, or depression or have a family history of suicide, bipolar illness, or depression
- have circulation problems in fingers and toes
- have kidney problems
- have or had repeated movements or sounds (tics) or Tourette’s syndrome, or have a family history of tics or Tourette’s syndrome
- are pregnant or plan to become pregnant. Lisdexamfetamine dimesylate chewable tablets may harm the unborn baby.
- are breastfeeding or plan to breastfeed. Lisdexamfetamine dimesylate passes into breast milk. You should not breastfeed during treatment with lisdexamfetamine dimesylate chewable tablets. Talk to your healthcare provider about the best way to feed the baby during treatment with lisdexamfetamine dimesylate chewable tablets.
Tell your healthcare provider about all the medicines that you or your child take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Lisdexamfetamine dimesylate chewable tablets can affect the way other medicines work and other medicines may affect how lisdexamfetamine dimesylate chewable tablets work. Taking lisdexamfetamine dimesylate chewable tablets with other medicines can cause serious side effects. Sometimes the doses of other medicines will need to be changed while taking lisdexamfetamine dimesylate chewable tablets.
Especially tell your healthcare provider if you or your child take:
- selective serotonin reuptake inhibitors (SSRIs)
- medicines used to treat migraine headaches called triptans
- lithium
- tramadol
- buspirone
- serotonin norepinephrine reuptake inhibitors (SNRIs)
- tricyclic antidepressants
- fentanyl
- tryptophan
- St. John’s Wort
Keep a list of all medicines to show your healthcare provider and pharmacist when you get a new medicine. Your healthcare provider will decide if lisdexamfetamine dimesylate chewable tablets can be taken with other medicines.
Do not start any new medicine during treatment with lisdexamfetamine dimesylate chewable tablets without talking to your healthcare provider first.
How should lisdexamfetamine dimesylate chewable tablets be taken?
- Take lisdexamfetamine dimesylate chewable tablets exactly as prescribed by your healthcare provider.
- Your healthcare provider may change the dose if needed.
- Take lisdexamfetamine dimesylate chewable tablets 1 time each day in the morning with or without food.
- Lisdexamfetamine dimesylate chewable tablets come in chewable tablets.
Taking Lisdexamfetamine Dimesylate Chewable Tablets:
- Chew lisdexamfetamine dimesylate chewable tablets completely before swallowing.
If you or your child take too many lisdexamfetamine dimesylate chewable tablets, call your healthcare provider or Poison Help line at 1-800-222-1222 or go to the nearest hospital emergency room right away.
What are the possible side effects of lisdexamfetamine dimesylate chewable tablets?
Lisdexamfetamine dimesylate chewable tablets may cause serious side effects, including:
- See “What is the most important information I should know about lisdexamfetamine dimesylate chewable tablets?”
- Slowing of growth (height and weight) in children. Children should have their height and weight checked often during treatment with lisdexamfetamine dimesylate chewable tablets. Lisdexamfetamine dimesylate chewable tablets treatment may be stopped if your child is not growing or gaining weight.
- Circulation problems in fingers and toes (Peripheral vasculopathy, including Raynaud’s phenomenon).
Signs and symptoms may include:
- Fingers or toes may feel numb, cool, painful
- Fingers or toes may change color from pale, to blue, to red
Tell your healthcare provider if you or your child have numbness, pain, skin color change, or sensitivity to temperature in your fingers or toes.
Call your healthcare provider right away if you or your child have any signs of unexplained wounds appearing on fingers or toes during treatment with lisdexamfetamine dimesylate chewable tablets.
- New or worsening tics or worsening Tourette’s syndrome. Tell your healthcare provider if you or your child get any new or worsening tics or worsening Tourette’s syndrome during treatment with lisdexamfetamine dimesylate chewable tablets.
- Serotonin Syndrome. A potentially life-threatening problem called serotonin syndrome may happen when lisdexamfetamine dimesylate chewable tablets are taken with certain other medicines. Stop taking lisdexamfetamine dimesylate chewable tablets and call your healthcare provider or go to the nearest hospital emergency room right away if you or your child develop any of the following signs and symptoms of serotonin syndrome:
- agitation
- flushing
- coma
- loss of coordination
- dizziness
- seeing or hearing things that are not real (hallucination)
- high body temperature (hyperthermia)
- fast heartbeat
- seizures
- sweating
- confusion
- tremors, stiff muscles, or muscle twitching
- changes in blood pressure
- nausea, vomiting, diarrhea
The most common side effects of lisdexamfetamine dimesylate chewable tablets in children 6 to 17 years old and adults with ADHD include:
- loss of appetite (anorexia)
- decreased appetite
- diarrhea
- dry mouth
- trouble sleeping
- stomach pain
- anxiety
- weight loss
- dizziness
- irritability
- nausea
- vomiting
The most common side effects of lisdexamfetamine dimesylate chewable tablets in adults with BED include:
- dry mouth
- decreased appetite
- constipation
- anxiety
- trouble sleeping
- increased heart rate
- feeling jittery
These are not all the possible side effects of lisdexamfetamine dimesylate chewable tablets.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store lisdexamfetamine dimesylate chewable tablets?
- Store lisdexamfetamine dimesylate chewable tablets in a safe place (like a locked cabinet) and in a tightly closed container at room temperature between 68° to 77°F (20° to 25°C).
- Protect lisdexamfetamine dimesylate chewable tablets from light.
- Dispose of remaining, unused, or expired lisdexamfetamine dimesylate chewable tablets by a medicine take-back program at a U.S. Drug Enforcement Administration (DEA) authorized collection site. If no take-back program or DEA authorized collector is available, mix lisdexamfetamine dimesylate chewable tablets with an undesirable, nontoxic substance such as dirt, cat litter, or used coffee grounds to make it less appealing to children and pets. Place the mixture in a container such as a sealed plastic bag and throw away (discard) lisdexamfetamine dimesylate chewable tablets in the household trash. Visit www.fda.gov/drugdisposal for additional information on disposal of unused medicines.
Keep lisdexamfetamine dimesylate chewable tablets and all medicines out of the reach of children.
General information about the safe and effective use of lisdexamfetamine dimesylate chewable tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use lisdexamfetamine dimesylate chewable tablets for a condition for which it was not prescribed. Do not give lisdexamfetamine dimesylate chewable tablets to other people, even if they have the same symptoms that you have. It may harm them and it is against the law. You can ask your pharmacist or healthcare provider for information about lisdexamfetamine dimesylate chewable tablets that is written for healthcare professionals.
What are the ingredients in lisdexamfetamine dimesylate chewable tablets?
Active Ingredient: lisdexamfetamine dimesylate
Chewable Tablets Inactive Ingredients: colloidal silicon dioxide, croscarmellose sodium, guar gum, magnesium stearate, mannitol, microcrystalline cellulose, sucralose, artificial strawberry flavor.
Manufactured for: SpecGx LLC, Webster Groves, MO 63119 USA
For more information, go to www.mallinckrodt.com or call 1-800-778-7898.
Mallinckrodt™
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: 05/2024
MG20L05
Close -
PRINCIPAL DISPLAY PANEL - 10 mg Chewable TabletsNDC 0406-5124-01 - 100 Chewable Tablets - Lisdexamfetamine Dimesylate - Chewable Tablets - CII - 10 mg - PHARMACIST: Dispense the Medication Guide provided separately to each patient. Chew tablets ...
NDC 0406-5124-01
100 Chewable Tablets
Lisdexamfetamine Dimesylate
Chewable TabletsCII
10 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
Chew tablets completely before swallowing.
Do not swallow tablets whole.Rx only
Mallinckrodt™
L00L10
Rev05/2024Close
-
PRINCIPAL DISPLAY PANEL - 20 mg Chewable TabletsNDC 0406-5125-01 - 100 Chewable Tablets - Lisdexamfetamine Dimesylate - Chewable Tablets - CII - 20 mg - PHARMACIST: Dispense the Medication Guide provided separately to each patient. Chew tablets ...
NDC 0406-5125-01
100 Chewable Tablets
Lisdexamfetamine Dimesylate
Chewable TabletsCII
20 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
Chew tablets completely before swallowing.
Do not swallow tablets whole.Rx only
Mallinckrodt™
L00L11
Rev 05/2024Close
-
PRINCIPAL DISPLAY PANEL - 30 mg Chewable TabletsNDC 0406-5126-01 - 100 Chewable Tablets - Lisdexamfetamine Dimesylate - Chewable Tablets - CII - 30 mg - PHARMACIST: Dispense the Medication Guide provided separately to each patient. Chew tablets ...
NDC 0406-5126-01
100 Chewable Tablets
Lisdexamfetamine Dimesylate
Chewable TabletsCII
30 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
Chew tablets completely before swallowing.
Do not swallow tablets whole.Rx only
Mallinckrodt™
L00L12
Rev 05/2024Close
-
PRINCIPAL DISPLAY PANEL - 40 mg Chewable TabletsNDC 0406-5127-01 - 100 Chewable Tablets - Lisdexamfetamine Dimesylate - Chewable Tablets - CII - 40 mg - PHARMACIST: Dispense the Medication Guide provided separately to each patient. Chew tablets ...
NDC 0406-5127-01
100 Chewable Tablets
Lisdexamfetamine Dimesylate
Chewable TabletsCII
40 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
Chew tablets completely before swallowing.
Do not swallow tablets whole.Rx only
Mallinckrodt™
L00L13
Rev 12/2024Close
-
PRINCIPAL DISPLAY PANEL - 50 mg Chewable TabletsNDC 0406-5128-01 - 100 Chewable Tablets - Lisdexamfetamine Dimesylate - Chewable Tablets - CII - 50 mg - PHARMACIST: Dispense the Medication Guide provided separately to each patient. Chew tablets ...
NDC 0406-5128-01
100 Chewable Tablets
Lisdexamfetamine Dimesylate
Chewable TabletsCII
50 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
Chew tablets completely before swallowing.
Do not swallow tablets whole.Rx only
Mallinckrodt™
L00L14
Rev 12/2024Close
-
PRINCIPAL DISPLAY PANEL - 60 mg Chewable TabletsNDC 0406-5129-01 - 100 Chewable Tablets - Lisdexamfetamine Dimesylate - Chewable Tablets - CII - 60 mg - PHARMACIST: Dispense the Medication Guide provided separately to each patient. Chew tablets ...
NDC 0406-5129-01
100 Chewable Tablets
Lisdexamfetamine Dimesylate
Chewable TabletsCII
60 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
Chew tablets completely before swallowing.
Do not swallow tablets whole.Rx only
Mallinckrodt™
L00L15
Rev 12/2024Close
-
INGREDIENTS AND APPEARANCEProduct Information
LISDEXAMFETAMINE DIMESYLATE lisdexamfetamine dimesylate tablet, chewable Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0406-5124 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LISDEXAMFETAMINE DIMESYLATE (UNII: SJT761GEGS) (LISDEXAMFETAMINE - UNII:H645GUL8KJ) LISDEXAMFETAMINE DIMESYLATE 10 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) GUAR GUM (UNII: E89I1637KE) SUCRALOSE (UNII: 96K6UQ3ZD4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (White to off-white) Score no score Shape ROUND Size 7mm Flavor STRAWBERRY Imprint Code M;10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0406-5124-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/17/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218850 12/17/2024 LISDEXAMFETAMINE DIMESYLATE lisdexamfetamine dimesylate tablet, chewable Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0406-5125 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LISDEXAMFETAMINE DIMESYLATE (UNII: SJT761GEGS) (LISDEXAMFETAMINE - UNII:H645GUL8KJ) LISDEXAMFETAMINE DIMESYLATE 20 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) GUAR GUM (UNII: E89I1637KE) SUCRALOSE (UNII: 96K6UQ3ZD4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (White to off-white) Score no score Shape HEXAGON (6 sided) Size 9mm Flavor STRAWBERRY Imprint Code M;20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0406-5125-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/17/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218850 12/17/2024 LISDEXAMFETAMINE DIMESYLATE lisdexamfetamine dimesylate tablet, chewable Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0406-5126 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LISDEXAMFETAMINE DIMESYLATE (UNII: SJT761GEGS) (LISDEXAMFETAMINE - UNII:H645GUL8KJ) LISDEXAMFETAMINE DIMESYLATE 30 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) GUAR GUM (UNII: E89I1637KE) SUCRALOSE (UNII: 96K6UQ3ZD4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (White to off-white) Score no score Shape TRIANGLE Size 10mm Flavor STRAWBERRY Imprint Code M;30 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0406-5126-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/17/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218850 12/17/2024 LISDEXAMFETAMINE DIMESYLATE lisdexamfetamine dimesylate tablet, chewable Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0406-5127 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LISDEXAMFETAMINE DIMESYLATE (UNII: SJT761GEGS) (LISDEXAMFETAMINE - UNII:H645GUL8KJ) LISDEXAMFETAMINE DIMESYLATE 40 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) GUAR GUM (UNII: E89I1637KE) SUCRALOSE (UNII: 96K6UQ3ZD4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (White to off-white) Score no score Shape CAPSULE Size 15mm Flavor STRAWBERRY Imprint Code M;40 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0406-5127-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/17/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218850 12/17/2024 LISDEXAMFETAMINE DIMESYLATE lisdexamfetamine dimesylate tablet, chewable Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0406-5128 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LISDEXAMFETAMINE DIMESYLATE (UNII: SJT761GEGS) (LISDEXAMFETAMINE - UNII:H645GUL8KJ) LISDEXAMFETAMINE DIMESYLATE 50 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) GUAR GUM (UNII: E89I1637KE) SUCRALOSE (UNII: 96K6UQ3ZD4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (White to off-white) Score no score Shape SQUARE Size 12mm Flavor STRAWBERRY Imprint Code M;50 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0406-5128-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/17/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218850 12/17/2024 LISDEXAMFETAMINE DIMESYLATE lisdexamfetamine dimesylate tablet, chewable Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0406-5129 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LISDEXAMFETAMINE DIMESYLATE (UNII: SJT761GEGS) (LISDEXAMFETAMINE - UNII:H645GUL8KJ) LISDEXAMFETAMINE DIMESYLATE 60 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) GUAR GUM (UNII: E89I1637KE) SUCRALOSE (UNII: 96K6UQ3ZD4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (White to off-white) Score no score Shape DIAMOND Size 15mm Flavor STRAWBERRY Imprint Code M;60 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0406-5129-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/17/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218850 12/17/2024 Labeler - SpecGx LLC (080679498) Establishment Name Address ID/FEI Business Operations SpecGx LLC 957414238 analysis(0406-5124, 0406-5125, 0406-5126, 0406-5127, 0406-5128, 0406-5129) , manufacture(0406-5124, 0406-5125, 0406-5126, 0406-5127, 0406-5128, 0406-5129) , pack(0406-5124, 0406-5125, 0406-5126, 0406-5127, 0406-5128, 0406-5129)
CloseEstablishment Name Address ID/FEI Business Operations SpecGx LLC 828788054 manufacture(0406-5124, 0406-5125, 0406-5126, 0406-5127, 0406-5128, 0406-5129)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
LISDEXAMFETAMINE DIMESYLATE tablet, chewable
Number of versions: 1
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Dec 26, 2024 | 4 (current) | download |
RxNorm
LISDEXAMFETAMINE DIMESYLATE tablet, chewable
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 1871456 | lisdexamfetamine dimesylate 40 MG Chewable Tablet | PSN |
| 2 | 1871456 | lisdexamfetamine dimesylate 40 MG Chewable Tablet | SCD |
| 3 | 1871460 | lisdexamfetamine dimesylate 30 MG Chewable Tablet | PSN |
| 4 | 1871460 | lisdexamfetamine dimesylate 30 MG Chewable Tablet | SCD |
| 5 | 1871462 | lisdexamfetamine dimesylate 20 MG Chewable Tablet | PSN |
| 6 | 1871462 | lisdexamfetamine dimesylate 20 MG Chewable Tablet | SCD |
| 7 | 1871464 | lisdexamfetamine dimesylate 10 MG Chewable Tablet | PSN |
| 8 | 1871464 | lisdexamfetamine dimesylate 10 MG Chewable Tablet | SCD |
| 9 | 1871466 | lisdexamfetamine dimesylate 60 MG Chewable Tablet | PSN |
| 10 | 1871466 | lisdexamfetamine dimesylate 60 MG Chewable Tablet | SCD |
| 11 | 1871468 | lisdexamfetamine dimesylate 50 MG Chewable Tablet | PSN |
| 12 | 1871468 | lisdexamfetamine dimesylate 50 MG Chewable Tablet | SCD |
Get Label RSS Feed for this Drug
LISDEXAMFETAMINE DIMESYLATE tablet, chewable
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=3a79b2ab-4131-4978-99a0-0119df57d5fe
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
LISDEXAMFETAMINE DIMESYLATE tablet, chewable
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 0406-5124-01 |
| 2 | 0406-5125-01 |
| 3 | 0406-5126-01 |
| 4 | 0406-5127-01 |
| 5 | 0406-5128-01 |
| 6 | 0406-5129-01 |






