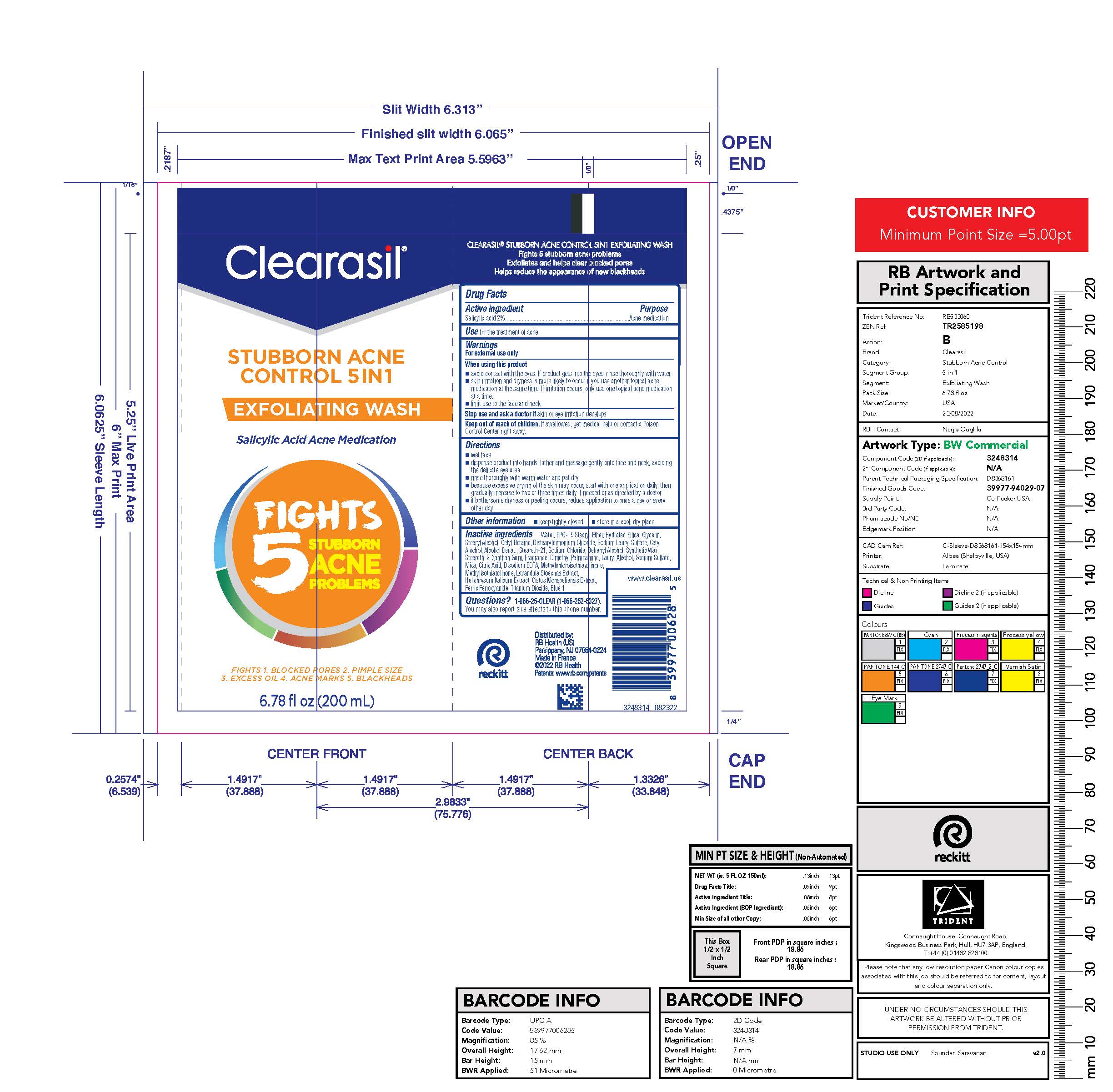
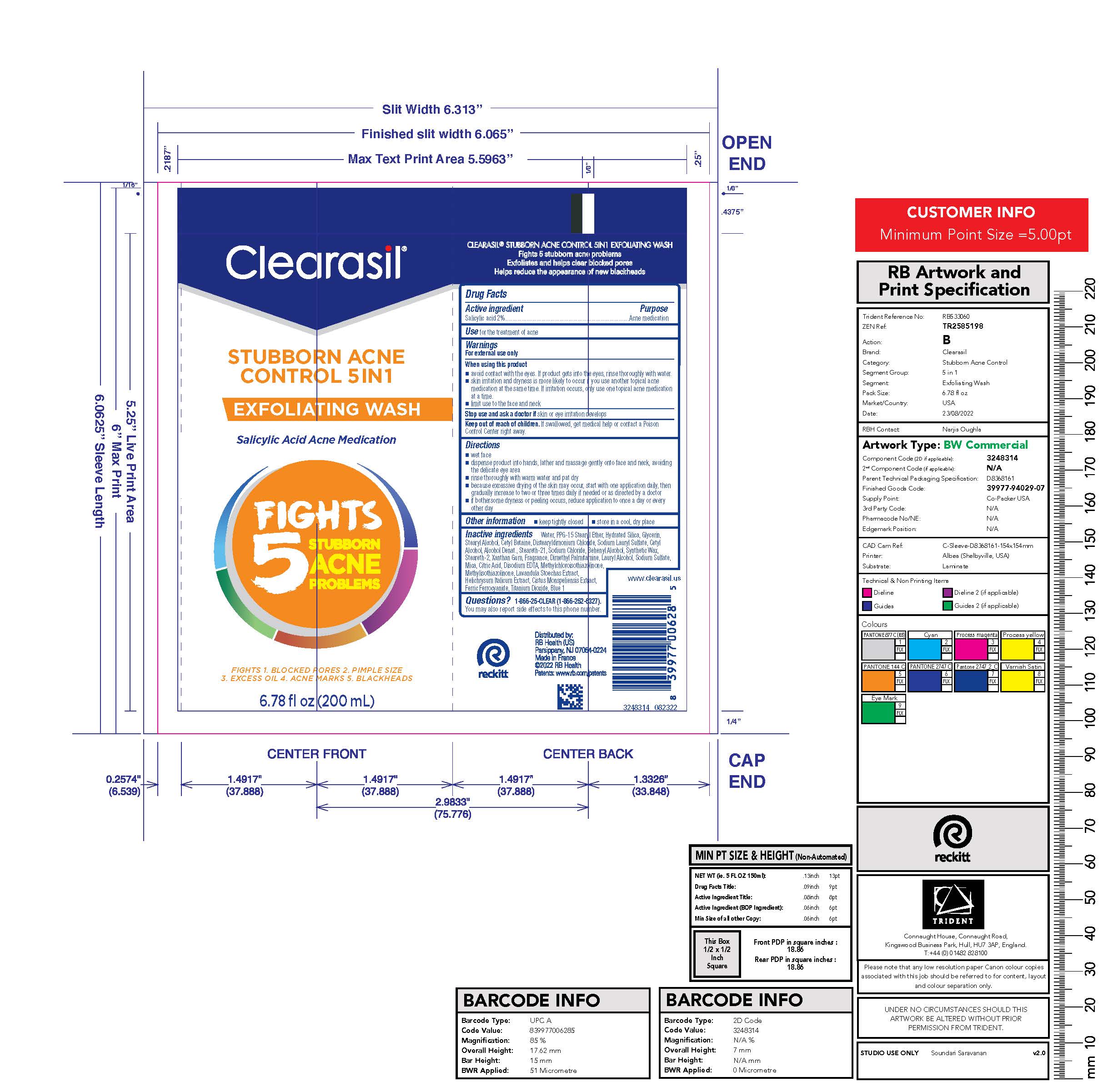
Label: CLEARASIL STUBBORN ACNE CONTROL 5IN1 EXFOLIATING WASH- salicylic acid lotion
- NDC Code(s): 63824-436-01
- Packager: RB Health (US) LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
When using this product
- avoid contact with the eyes. If product gets into the eyes, rinse thoroughly with water.
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- limit use to the face and neck
-
Directions
- wet face
- dispense product into hands, lather and massage gently onto face and neck, avoiding the delicate eye area
- rinse thoroughly with warm water one too three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- Other information
-
Inactive ingredients
Water, PPG-15 Stearyl Ether, Hydrated Silica, Glycerin, Stearyl Alcohol, Cetyl Betaine, Distearyldimonium Chloride, Sodium Lauryl Sulfate, Cetyl Alcohol, Alcohol Denat., Steareth-21, Sodium Chloride, Behenyl Alcohol, Synthetic Wax, Steareth-2, Xanthan Gum, Fragrance, Dimethyl Palmitamine, Lauryl Alcohol, Sodium Sulfate, Mica, Citric Acid, Disodium EDTA, Methylchloroisothiazolinone, Methylisothiazolinone, Lavandula Stoechas Extract, Helicrysum Italicum Extract, Cistus Monspeliensis Extract, Ferric Ferrocyanide, Titanium Dioxide, Blue 1
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 200 mL Tube Label
-
INGREDIENTS AND APPEARANCE
CLEARASIL STUBBORN ACNE CONTROL 5IN1 EXFOLIATING WASH
salicylic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63824-436 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PPG-15 STEARYL ETHER (UNII: 1II18XLS1L) HYDRATED SILICA (UNII: Y6O7T4G8P9) GLYCERIN (UNII: PDC6A3C0OX) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) CETYL BETAINE (UNII: E945X08YA9) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CETYL ALCOHOL (UNII: 936JST6JCN) ALCOHOL (UNII: 3K9958V90M) STEARETH-21 (UNII: 53J3F32P58) SODIUM CHLORIDE (UNII: 451W47IQ8X) DOCOSANOL (UNII: 9G1OE216XY) STEARETH-2 (UNII: V56DFE46J5) XANTHAN GUM (UNII: TTV12P4NEE) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) HELICHRYSUM ITALICUM FLOWER (UNII: P62Y550X24) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) MICA (UNII: V8A1AW0880) FERRIC FERROCYANIDE (UNII: TLE294X33A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63824-436-01 200 mL in 1 TUBE; Type 0: Not a Combination Product 12/05/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/05/2017 Labeler - RB Health (US) LLC (081049410)