Label: MEDROL- methylprednisolone tablet
- NDC Code(s): 0009-0020-01, 0009-0022-01, 0009-0049-02, 0009-0056-02, view more
- Packager: Pharmacia & Upjohn Company LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
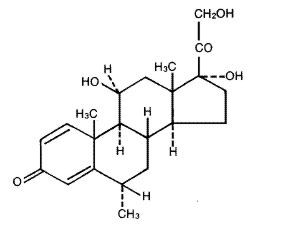
DESCRIPTIONMEDROL Tablets contain methylprednisolone which is a glucocorticoid. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the ...
-
INDICATIONS AND USAGEMEDROL Tablets are indicated in the following conditions: 1. Endocrine Disorders - Primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the first choice; synthetic ...
-
CONTRAINDICATIONSSystemic fungal infections and known hypersensitivity to components.
-
WARNINGSIn patients on corticosteroid therapy subjected to unusual stress, increased dosage of rapidly acting corticosteroids before, during, and after the stressful situation is ...
-
PRECAUTIONSGeneral Precautions - Drug-induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after ...
-
ADVERSE REACTIONSFluid and Electrolyte Disturbances - • Sodium retention - • Congestive heart failure in susceptible patients - • Hypertension - • Fluid retention - • Potassium loss - • Hypokalemic ...
-
DOSAGE AND ADMINISTRATIONThe initial dosage of MEDROL Tablets may vary from 4 mg to 48 mg of methylprednisolone per day depending on the specific disease entity being treated. In situations of less severity lower doses ...
-
HOW SUPPLIEDMEDROL Tablets are available in the following strengths and package sizes: 2 mg (white, elliptical, scored, imprinted MEDROL 2) Bottles of 100 ...
-
REFERENCES1 Fekety R. Infections associated with corticosteroids and immunosuppressive therapy. In: Gorbach SL, Bartlett JG, Blacklow NR, eds. Infectious Diseases. Philadelphia: WBSaunders Company ...
-
SPL UNCLASSIFIED SECTIONThis product's label may have been updated. For current full prescribing information, please visit www.pfizer.com. LAB-0157-11.0 - Revised March 2025
-
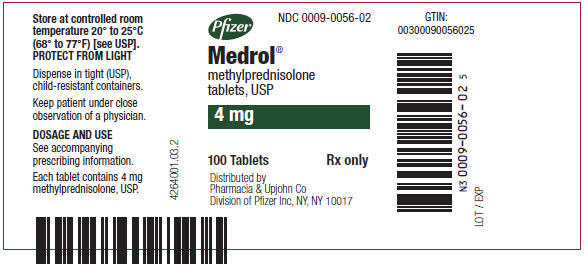
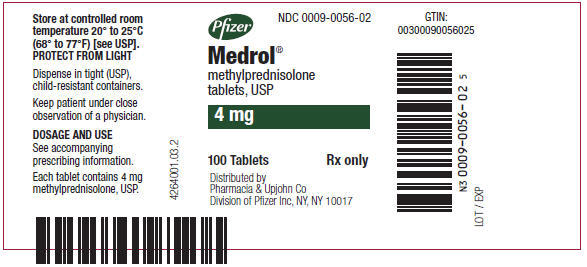
PRINCIPAL DISPLAY PANEL - 4 mg Tablet Bottle LabelPfizer - NDC 0009-0056-02 - Medrol® methylprednisolone - tablets, USP - 4 mg - 100 Tablets - Rx only - Distributed by - Pharmacia & Upjohn Co - Division of Pfizer Inc, NY, NY 10017
-
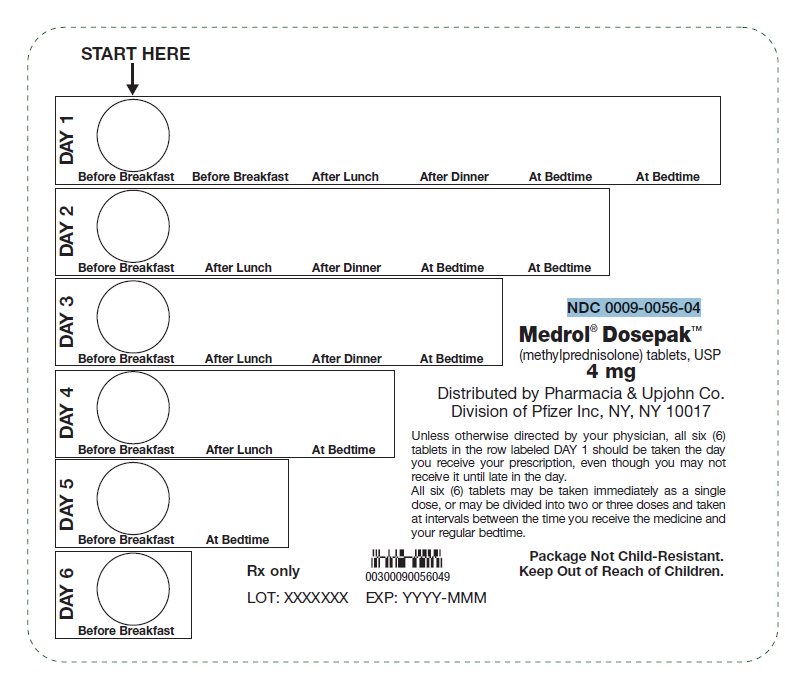
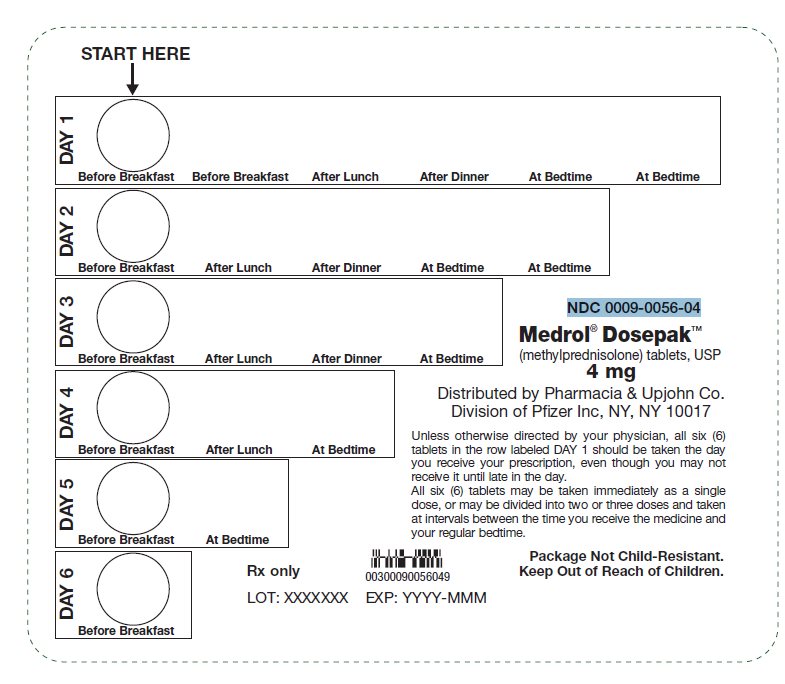
PRINCIPAL DISPLAY PANEL - 4 mg Tablet Dose PackSTART HERE - DAY 1 - Before Breakfast - Before Breakfast - After Lunch - After Dinner - At Bedtime - At Bedtime - DAY 2 - Before Breakfast - After Lunch - After Dinner - At Bedtime - At Bedtime - DAY 3 - Before ...
-
PRINCIPAL DISPLAY PANEL - 4 mg Tablet Dose Pack CartonNDC 0009-0056-04 - Pfizer - Medrol® Dosepak™ 4 mg - (methylprednisolone) tablets, USP - Unit of Use - One blister containing 21 tablets - Rx only
-
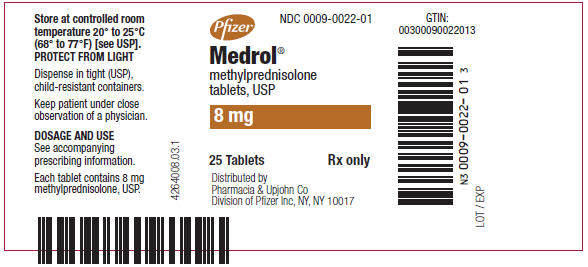
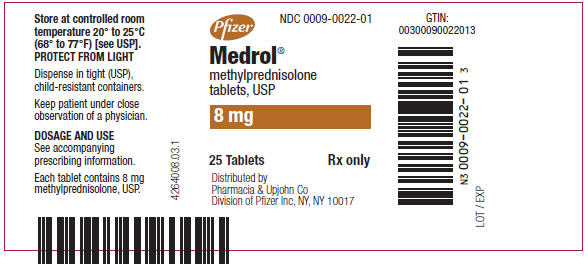
PRINCIPAL DISPLAY PANEL - 8 mg Tablet Bottle LabelPfizer - NDC 0009-0022-01 - Medrol® methylprednisolone - tablets, USP - 8 mg - 25 Tablets - Rx only - Distributed by - Pharmacia & Upjohn Co - Division of Pfizer Inc, NY, NY 10017
-
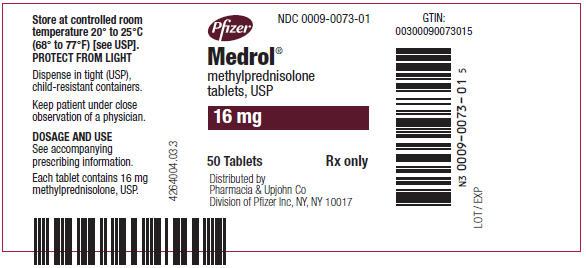
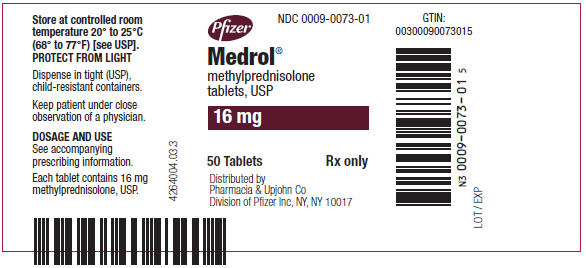
PRINCIPAL DISPLAY PANEL - 16 mg Tablet Bottle LabelPfizer - NDC 0009-0073-01 - Medrol® methylprednisolone - tablets, USP - 16 mg - 50 Tablets - Rx only - Distributed by - Pharmacia & Upjohn Co - Division of Pfizer Inc, NY, NY 10017
-
PRINCIPAL DISPLAY PANEL - 32 mg Tablet Bottle LabelPfizer - NDC 0009-0176-01 - Medrol® methylprednisolone - tablets, USP - 32 mg - 25 Tablets - Rx only - Distributed by - Pharmacia & Upjohn Co - Division of Pfizer Inc, NY, NY 10017
-
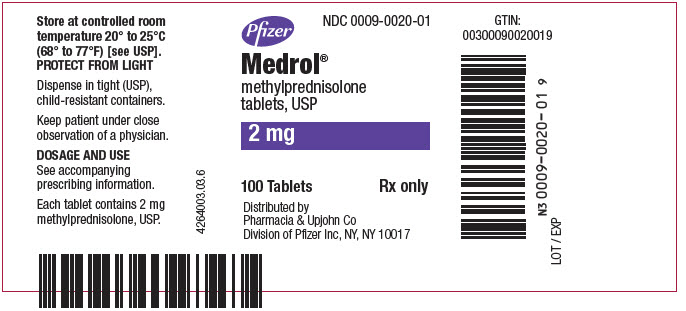
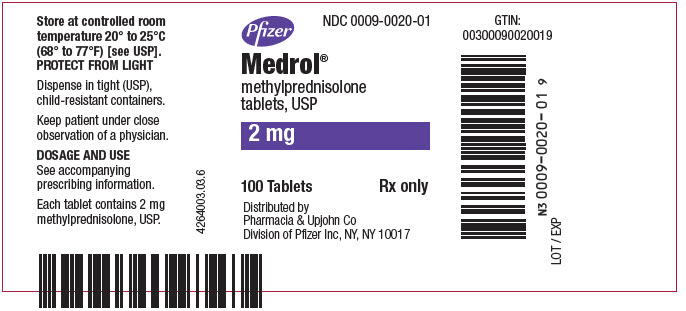
PRINCIPAL DISPLAY PANEL - 2 mg Tablet Bottle LabelPfizer - NDC 0009-0020-01 - Medrol® methylprednisolone - tablets, USP - 2 mg - 100 Tablets - Rx only - Distributed by - Pharmacia & Upjohn Co - Division of Pfizer Inc, NY, NY 10017
-
INGREDIENTS AND APPEARANCEProduct Information