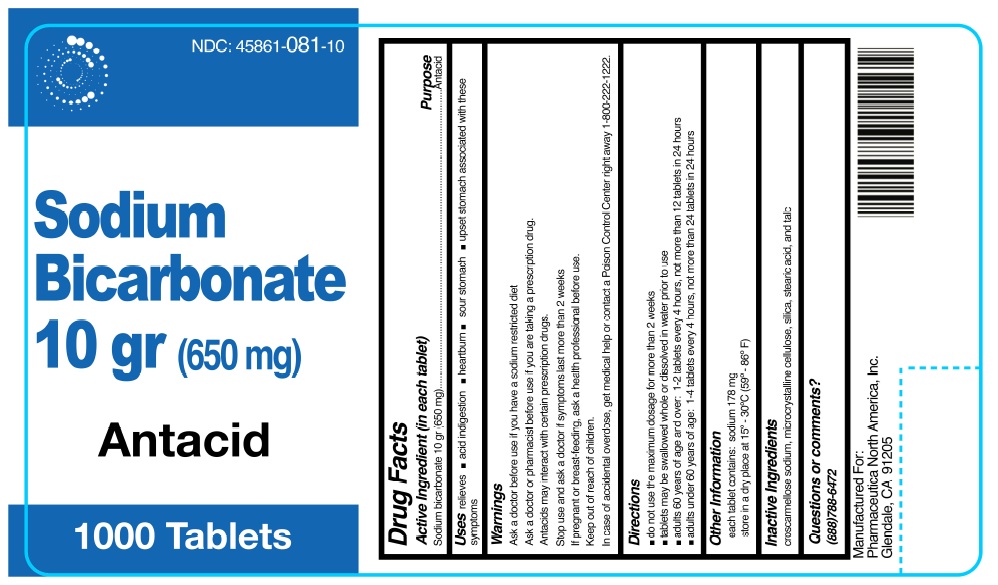
Label: SODIUM BICARBONATE 10GR (650MG)- sodium bicarbonate tablet
- NDC Code(s): 45861-081-10
- Packager: Pharmaceutica North America, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 14, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient (in each tablet)Sodium bicarbonate 10 gr (650 mg)
-
Purpose
Antacid
-
Uses
relieves: • acid indigestion - • heartburn - • sour stomach - • upset stomach associated with these symptoms
-
Warnings
Ask a doctor before use if you havea sodium restricted diet. Ask a doctor or pharmacist before use if you aretaking a prescription drug. Antacids may interact with certain prescription ...
- KEEP OUT OF REACH OF CHILDREN
-
Directions
• do not use the maximum dosage for more than 2 weeks - • tablets may be swallowed whole or dissolved in water prior to use - • adults 60 years of age and over: 1-2 tablets every 4 ...
-
Other information
• each tablet contains: sodium 178 mg - • store in a dry place at 15° – 30°C (59° – 86°F).
-
Inactive Ingredients
croscarmellose sodium, microcrystalline cellulose, silica, stearic acid, talc.
- Questions or comments? (888)788-6472
-
Product label
-
INGREDIENTS AND APPEARANCEProduct Information

