Label: REMIFENTANIL HYDROCHLORIDE injection, powder, lyophilized, for solution
-
NDC Code(s):
75834-230-01,
75834-230-10,
75834-231-01,
75834-231-10, view more75834-232-01, 75834-232-10
- Packager: Nivagen Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use REMIFENTANIL HYDROCHLORIDE FOR INJECTION safely and effectively. See full prescribing information for REMIFENTANIL HYDROCHLORIDE FOR ...These highlights do not include all the information needed to use REMIFENTANIL HYDROCHLORIDE FOR INJECTION safely and effectively. See full prescribing information for REMIFENTANIL HYDROCHLORIDE FOR INJECTION.
Remifentanil hydrochloride for injection, for intravenous use, CII
Initial U.S. Approval: 1996
WARNING: SERIOUS AND LIFE-THREATENING RISKS FROM USE OF REMIFENTANIL HYDROCHLORIDE FOR INJECTION
See full prescribing information for complete boxed warning.
- Remifentanil hydrochloride for injection exposes users to the risks of addiction, abuse, and misuse. Assess patient's risk before prescribing and reassess regularly for the development of these behaviors and conditions.(5.1)
- Serious, life-threatening, or fatal respiratory depression may occur with use of Remifentanil hydrochloride for injection, especially during initiation or following a dosage increase. To reduce the risk of respiratory depression, proper dosing and titration of Remifentanil hydrochloride for injection are essential. (5.2)
- Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing for use in patients for whom alternative treatment options are inadequate. (5.3, 7)
INDICATIONS AND USAGE
Remifentanil hydrochloride for injection is an opioid agonist indicated for intravenous administration:
- As an analgesic agent for use during the induction and maintenance of general anesthesia for inpatient and outpatient procedures. (1)
- For continuation as an analgesic into the immediate postoperative period in adult patients under the direct supervision of an anesthesia practitioner in a postoperative anesthesia care unit or intensive care setting. (1)
- As an analgesic component of monitored anesthesia care in adult patients. (1)
DOSAGE AND ADMINISTRATION
- Monitor patients closely for respiratory depression when initiating therapy and following dosage increases and adjust the dosage accordingly. (2.1)
- Initial Dosage in Adults: See full prescribing information for recommended doses in adult patients. (2.2, 2.3)
- Initial Dosage in Pediatric Patients: See full prescribing information for recommended doses in pediatric patients. (2.2)
- Geriatric Patients: The starting doses should be decreased by 50% in elderly patients (> 65 years). (2.6)
DOSAGE FORMS AND STRENGTHS
For injection: 1 mg, 2 mg, and 5 mg for intravenous administration after reconstitution and dilution (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Life-Threatening Respiratory Depression: Monitor closely, particularly during initiation and titration. (5.2)
- Risks from Use as Postoperative Analgesia with Concomitant Benzodiazepines or other CNS Depressants: Hypotension, profound sedation, respiratory depression, coma, and death may result from the concomitant use of Remifentanil hydrochloride for injection with benzodiazepines or other CNS depressants (5.3)
- Opioid-Induced Hyperalgesia and Allodynia: Opioid-Induced Hyperalgesia (OIH) occurs when an opioid analgesic paradoxically causes an increase in pain, or an increase in sensitivity to pain. If OIH is suspected, carefully consider appropriately decreasing the dose of the current opioid analgesic or opioid rotation. (5.4)
- Serotonin Syndrome: Potentially life-threatening condition could result from concomitant serotonergic drug administration. Discontinue Remifentanil hydrochloride for injection if serotonin syndrome is suspected. (5.5)
- Administration: Continuous infusions of Remifentanil hydrochloride for injection should be administered only by an infusion device. (5.6)
- Skeletal Muscle Rigidity: is related to the dose and speed of administration. Muscle rigidity induced by Remifentanil hydrochloride for injection should be managed in the context of the patient's clinical condition. (5.7)
- Potential Inactivation by Nonspecific Esterases in Blood Products: Remifentanil hydrochloride for injection should not be administered into the same IV tubing with blood due to potential inactivation by nonspecific esterases in blood products. (5.8)
- Bradycardia: Monitor heart rate during dosage initiation and titration. It is responsive to ephedrine or anticholinergic drugs (5.9)
- Hypotension: Monitor blood pressure during dosage initiation and titration. It is responsive to decreases in the administration of Remifentanil hydrochloride for injection or to IV fluids or catecholamine administration (5.10)
- Intraoperative Awareness: Inoperative awareness has been reported in patients under 55 years of age when Remifentanil hydrochloride for injection has been administered with propofol infusion rates of ≤ 75 mcg/kg/min (5.11)
- Risks of Use in Spontaneously Breathing Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness: Monitor for sedation and respiratory depression. (5.12)
- Risks of Use in Patients with Biliary Tract Disease: Monitor patients with biliary tract disease, including acute pancreatitis, for worsening symptoms. (5.13)
- Increased Risk of Seizures in Patients with Seizure Disorders: Monitor patients with a history of seizure disorders for worsened seizure control during Remifentanil hydrochloride for injection therapy. (5.14)
- Rapid Offset of Action: Standard monitoring should be maintained in the postoperative period to ensure adequate recovery without stimulation. (5.15)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 1%) were respiratory depression, bradycardia, hypotension, and skeletal muscle rigidity. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Nivagen Pharmaceuticals, Inc. at 1-877-977-0687 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics: May reduce the analgesic effect of Remifentanil hydrochloride for injection and/or precipitate withdrawal symptoms. If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment. (7)
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause fetal harm. (8.1)
- Labor or Delivery: Respiratory depression and other opioid effects may occur in newborns whose mothers are given Remifentanil hydrochloride for injection shortly before delivery. (8.1)
- Lactation: Infants exposed to Remifentanil hydrochloride for injection through breast milk should be monitored for excess sedation and respiratory depression. (8.2)
- Pediatric Use: Remifentanil hydrochloride for injection has not been studied in pediatric patients for use as a postoperative analgesic or as an analgesic component of monitored anesthesia care. (8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2024
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SERIOUS AND LIFE-THREATENING RISKS FROM USE OF REMIFENTANIL HYDROCHLORIDE FOR INJECTION
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
2.2 General Anesthesia
2.3 Continuation as an Analgesic into the Immediate Postoperative Period Under the Direct Supervision of an Anesthesia Practitioner
2.4 Analgesic Component of Monitored Anesthesia Care
2.5 Discontinuation
2.6 Dosage Modifications in Geriatric Patients
2.7 Dosage Modifications in Pediatric Patients
2.8 Dosage Modifications in Coronary Artery Bypass Surgery
2.9 Dosage Modifications in Obese Patients
2.10 Dosage Modifications in Preanesthetic Medication
2.11 Preparation for Administration
2.12 Compatibility and Stability
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Addiction, Abuse and Misuse
5.2 Life-Threatening Respiratory Depression
5.3 Risks from Use as Postoperative Analgesia with Concomitant Benzodiazepines or Other CNS Depressants
5.4 Opioid-Induced Hyperalgesia and Allodynia
5.5 Serotonin Syndrome with Concomitant Use of Serotonergic Drugs
5.6 Administration
5.7 Skeletal Muscle Rigidity
5.8 Potential Inactivation by Nonspecific Esterases in Blood Products
5.9 Bradycardia
5.10 Hypotension
5.11 Intraoperative Awareness
5.12 Risks of Use in Spontaneously Breathing Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness
5.13 Risks of Use in Patients with Biliary Tract Disease
5.14 Increased Risk of Seizures in Patients with Seizure Disorders
5.15 Rapid Offset of Action
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Use in Morbidly Obese Patients
8.7 Long-Term Use in the ICU
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Induction and Maintenance of General Anesthesia - Inpatient/Outpatient
14.2 Recovery
14.3 Spontaneous Ventilation Anesthesia
14.4 Pediatric Anesthesia
14.5 Coronary Artery Bypass Surgery
14.6 Neurosurgery
14.7 Continuation of Analgesic Use into the Immediate Postoperative Period
14.8 Monitored Anesthesia Care
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SERIOUS AND LIFE-THREATENING RISKS FROM USE OF REMIFENTANIL HYDROCHLORIDE FOR INJECTION
Addiction, Abuse, and Misuse
Because the use of Remifentanil hydrochloride for injection exposes patients and other users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death, assess each patient’s risk prior to prescribing and reassess all patients regularly for the development of these behaviors and conditions [see Warnings and Precautions (5.1)].
Life-Threatening Respiratory Depression
Serious, life-threatening, or fatal respiratory depression may occur with use of Remifentanil hydrochloride for injection, especially during initiation or following a dosage increase. To reduce the risk of respiratory depression, proper dosing and titration of Remifentanil hydrochloride for injection are essential [see Warnings and Precautions (5.2)].
Close
Risks From Concomitant Use With Benzodiazepines Or Other CNS Depressants
Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of Remifentanil hydrochloride for injection and benzodiazepines or other CNS depressants for use in patients for whom alternative treatment options are inadequate [see Warnings and Precautions (5.3), Drug Interactions (7)].
-
1 INDICATIONS AND USAGERemifentanil hydrochloride for injection is indicated for intravenous (IV) administration: As an analgesic agent for use during the induction and maintenance of general anesthesia for inpatient ...
Remifentanil hydrochloride for injection is indicated for intravenous (IV) administration:
- As an analgesic agent for use during the induction and maintenance of general anesthesia for inpatient and outpatient procedures.
- For continuation as an analgesic into the immediate postoperative period in adult patients under the direct supervision of an anesthesia practitioner in a postoperative anesthesia care unit or intensive care setting.
- As an analgesic component of monitored anesthesia care in adult patients.
Close -
2 DOSAGE AND ADMINISTRATION2.1 Important Dosage and Administration Instructions - Monitor patients closely for respiratory depression when initiating therapy and following dosage increases with Remifentanil hydrochloride ...
2.1 Important Dosage and Administration Instructions
Monitor patients closely for respiratory depression when initiating therapy and following dosage increases with Remifentanil hydrochloride for injection and adjust the dosage accordingly [see Warnings and Precautions (5.2)].
Remifentanil hydrochloride for injection is for IV use only. Continuous infusions of Remifentanil hydrochloride for injection should be administered only by an infusion device. The injection site should be close to the venous cannula and all IV tubing should be cleared at the time of discontinuation of infusion.
Remifentanil hydrochloride for injection should not be administered without dilution.
Consider an alternative to Remifentanil hydrochloride for injection for patients taking mixed agonist/antagonist and partial agonist opioid analgesics due to reduced analgesic effect or potential withdrawal symptoms. If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment. Discontinue Remifentanil hydrochloride for injection if patient is not responding appropriately to treatment.
Discard unused portion.
2.2 General Anesthesia
Remifentanil hydrochloride for injection is not recommended as the sole agent in general anesthesia because loss of consciousness cannot be assured and because of a high incidence of apnea, muscle rigidity, and tachycardia. Remifentanil hydrochloride for injection is synergistic with other anesthetics; therefore, clinicians may need to reduce doses of thiopental, propofol, isoflurane, and midazolam by up to 75% with the coadministration of Remifentanil hydrochloride for injection. The administration of Remifentanil hydrochloride for injection must be individualized based on the patient's response.
Induction of Anesthesia
Remifentanil hydrochloride for injection should be administered at an infusion rate of 0.5 to 1 mcg/kg/min with a hypnotic or volatile agent for the induction of anesthesia. If endotracheal intubation is to occur less than 8 minutes after the start of the infusion of Remifentanil hydrochloride for injection, then an initial dose of 1 mcg/kg may be administered over 30 to 60 seconds.
Remifentanil hydrochloride for injection should not be used as a sole agent for induction of anesthesia because loss of consciousness cannot be assured and because of a high incidence of apnea, muscle rigidity, and tachycardia.
Maintenance of Anesthesia
After endotracheal intubation, the infusion rate of Remifentanil hydrochloride for injection should be decreased in accordance with the dosing guidelines in Tables 1 (adults, predominately ASA physical status I, II, or III) and 2 (pediatric patients).
- Due to the fast onset and short duration of action of Remifentanil hydrochloride for injection, the rate of administration during anesthesia can be titrated upward in 25% to 100% increments in adult patients or up to 50% increments in pediatric patients, or downward in 25% to 50% decrements every 2 to 5 minutes to attain the desired level of μ-opioid effect.
- In response to light anesthesia or transient episodes of intense surgical stress, supplemental bolus doses of 1 mcg/kg may be administered every 2 to 5 minutes.
- At infusion rates > 1 mcg/kg/min, increases in the concomitant anesthetic agents should be considered to increase the depth of anesthesia. [See Clinical Pharmacology: Specific Populations: Pediatric Population (12.3) and Dosage and Administration, Table 2 (2.2).]
Table 1: Dosing Guidelines in Adults – General Anesthesia and Continuing as an Analgesic into the Postoperative Care Unit or Intensive Care Settinga
Phase
Continuous IV Infusion of Remifentanil hydrochloride for injection(mcg/kg/min)
Range of Infusion Dose Remifentanil hydrochloride for injection(mcg/kg/min)
Supplemental IV Bolus Dose of Remifentanil hydrochloride for injection
(mcg/kg)
Induction of Anesthesia (through intubation)
0.5 – 1a
Maintenance of anesthesia with:
Nitrous oxide (66%)
0.4
0.1 – 2
1
Isoflurane (0.4 to 1.5 MAC)
0.25
0.05 – 2
1
Propofol (100 to 200 mcg/kg/min)
0.25
0.05 – 2
1
Continuation as an analgesic into the immediate postoperative period
0.1
0.025 – 0.2
not recommended
a An initial dose of 1 mcg/kg may be administered over 30 to 60 seconds.
Table 2 summarizes the recommended doses in pediatric patients, predominantly ASA physical status I, II, or III. In pediatric patients, remifentanil was administered with nitrous oxide or nitrous oxide in combination with halothane, sevoflurane, or isoflurane. The use of atropine may blunt the potential for bradycardia that can occur upon administration of Remifentanil hydrochloride for injection.
Table 2: Dosing Guidelines in Pediatric Patients – Maintenance of Anesthesia
Phase
Continuous IV Infusion of Remifentanil hydrochloride for injection(mcg/kg/min)
Range of
Infusion Dose Remifentanil hydrochloride for injection(mcg/kg/min)
Supplemental IV Bolus Dose of Remifentanil hydrochloride for injection
(mcg/kg)
Maintenance of anesthesia in patients aged 1 to 12 years old witha:
Halothane (0.3 to 1.5 MAC)
0.25
0.05 – 1.3
1
Sevoflurane (0.3 to 1.5 MAC)
0.25
0.05 – 1.3
1
Isoflurane (0.4 to 1.5 MAC)
0.25
0.05 – 1.3
1
Maintenance of anesthesia for patients from birth to 2 months of age with:
Nitrous oxide (70%)b
0.4
0.4 – 1.0
1c
a An initial dose of 1 mcg/kg may be administered over 30 to 60 seconds.
b The clearance rate in neonates is highly variable, on average two times higher than in the young healthy adult population. Therefore, an increased infusion rate may be necessary to maintain adequate surgical anesthesia, and additional bolus doses may be required. The use of atropine may blunt the potential for bradycardia that can occur upon administration of Remifentanil hydrochloride for injection. [See Clinical Pharmacology: Specific Populations: Pediatric Population (12.3) and Clinical Studies (14.4).]
c Boluses of 1 mcg/kg were studied in ASA 1 and 2, full-term patients weighing at least 2500 gm, undergoing pyloromyotomy who received pretreatment with atropine. Neonates receiving supplementation with potent inhalation agents or neuraxial anesthesia, those with significant co-morbidities or undergoing significant fluid shifts, or those who have not been pretreated with atropine, may require smaller bolus doses to avoid hypotension and/or bradycardia.
2.3 Continuation as an Analgesic into the Immediate Postoperative Period Under the Direct Supervision of an Anesthesia Practitioner
Infusions of Remifentanil hydrochloride for injection may be continued into the immediate postoperative period for select patients for whom later transition to longer acting analgesics may be desired.
- Remifentanil hydrochloride for injection has not been studied in pediatric patients for use in the immediate postoperative period.
- The use of bolus injections of Remifentanil hydrochloride for injection to treat pain during the postoperative period is not recommended.
- When used as an IV analgesic in the immediate postoperative period, Remifentanil hydrochloride for injection should be initially administered by continuous infusion at a rate of 0.1 mcg/kg/min.
- The infusion rate may be adjusted every 5 minutes in 0.025 mcg/kg/min increments to balance the patient's level of analgesia and respiratory rate.
- Infusion rates greater than 0.2 mcg/kg/min are associated with respiratory depression (respiratory rate less than 8 breaths/min).
Due to the rapid offset of action of Remifentanil hydrochloride for injection, no residual analgesic activity will be present within 5 to 10 minutes after discontinuation. For patients undergoing surgical procedures where postoperative pain is generally anticipated, alternative analgesics should be administered prior to discontinuation of Remifentanil hydrochloride for injection. The choice of analgesic should be appropriate for the patient's surgical procedure and the level of follow-up care [see Clinical Studies (14)].
2.4 Analgesic Component of Monitored Anesthesia Care
It is strongly recommended that supplemental oxygen be supplied to the patient whenever Remifentanil hydrochloride for injection is administered.
- Remifentanil hydrochloride for injection has not been studied for use in children in monitored anesthesia care.
Single Dose
A single IV dose of 0.5 to 1 mcg/kg over 30 to 60 seconds of Remifentanil hydrochloride for injection may be given 90 seconds before the placement of the local or regional anesthetic block [see Warnings and Precautions (5.7)].
Continuous Infusion
When used alone as an IV analgesic component of monitored anesthesia care, Remifentanil hydrochloride for injection should be initially administered by continuous infusion at a rate of 0.1 mcg/kg/min beginning 5 minutes before placement of the local or regional anesthetic block.
- Because of the risk for hypoventilation, the infusion rate of Remifentanil hydrochloride for injection should be decreased to 0.05 mcg/kg/min following placement of the block.
- Thereafter, rate adjustments of 0.025 mcg/kg/min at 5 minute intervals may be used to balance the patient's level of analgesia and respiratory rate.
- Rates greater than 0.2 mcg/kg/min are generally associated with respiratory depression (respiratory rates less than 8 breaths/min).
- Bolus doses of Remifentanil hydrochloride for injection administered simultaneously with a continuous infusion of Remifentanil hydrochloride for injection to spontaneously breathing patients are not recommended.
Table 3 summarizes the recommended doses for monitored anesthesia care in adult patients, predominately ASA physical status I, II, or III.
Table 3: Dosing Guidelines in Adults – Monitored Anesthesia Care
Method
Timing
Remifentanil hydrochloride for injection Alone
Remifentanil hydrochloride for injection + 2 mg Midazolam
Single IV Dose
Given 90 seconds before local anesthetic
1 mcg/kg over 30 to 60 seconds
0.5 mcg/kg over 30 to 60 seconds
Continuous IV Infusion
Beginning 5 minutes before local anesthetic
0.1 mcg/kg/min
0.05 mcg/kg/min
After local anesthetic
0.05 mcg/kg/min (Range: 0.025 to 0.2 mcg/kg/min)
0.025 mcg/kg/min (Range: 0.025 to 0.2 mcg/kg/min)
2.5 Discontinuation
Upon discontinuation of Remifentanil hydrochloride for injection, the IV tubing should be cleared to prevent the inadvertent administration of Remifentanil hydrochloride for injection at a later time.
For patients undergoing surgical procedures where postoperative pain is generally anticipated, alternative analgesics should be administered prior to discontinuation of Remifentanil hydrochloride for injection. The choice of analgesic should be appropriate for the patient's surgical procedure and the level of follow-up care [see Clinical Studies (14)].
2.6 Dosage Modifications in Geriatric Patients
The starting doses of Remifentanil hydrochloride for injection should be decreased by 50% in elderly patients (> 65 years). Remifentanil hydrochloride for injection should then be cautiously titrated to effect [see Use in Specific Populations (8.5)].
2.7 Dosage Modifications in Pediatric Patients
See Table 2 for dosing recommendations for use of Remifentanil hydrochloride for injection in pediatric patients from birth to 12 years of age for maintenance of anesthesia. [See Clinical Pharmacology: Specific Populations: Pediatric Population (12.3) and Dosage and Administration, Table 2 and Maintenance of Anesthesia (2.2).]
Remifentanil hydrochloride for injection has not been studied in pediatric patients for use in the immediate postoperative period or for use as a component of monitored anesthesia care.
2.8 Dosage Modifications in Coronary Artery Bypass Surgery
Table 4 summarizes the recommended doses for induction, maintenance, and continuation as an analgesic into the ICU in adult patients, predominantly ASA physical status III or IV. To avoid hypotension during the induction phase, it is important to consider the concomitant medication regimens. [See Clinical Studies: Coronary Artery Bypass Surgery (14.5).]
Table 4: Dosing Recommendationsa – Coronary Artery Bypass Surgery
Phase
Continuous IV Infusion of
Remifentanil hydrochloride for injection(mcg/kg/min)
Range of
Infusion Dose Remifentanil hydrochloride for injection (mcg/kg/min)
Supplemental IV Bolus Dose of Remifentanil hydrochloride for injection(mcg/kg)
Induction of Anesthesia (through intubation)
1
Maintenance of Anesthesia
1
0.125 to 4
0.5 to 1
Continuation as an analgesic into ICU
1
0.05 to 1
aSee Clinical Studies: Coronary Artery Bypass Surgery subsection (14.5) for concomitant medication regimens.
2.9 Dosage Modifications in Obese Patients
The starting doses of Remifentanil hydrochloride for injection should be based on ideal body weight (IBW) in obese patients (greater than 30% over their IBW) [see Use in Specific Populations (8.6)].
2.10 Dosage Modifications in Preanesthetic Medication
The need for premedication and the choice of anesthetic agents must be individualized. In clinical studies, patients who received Remifentanil hydrochloride for injection frequently received a benzodiazepine premedication.
2.11 Preparation for Administration
To reconstitute solution, add 1 mL of diluent per mg of remifentanil. Shake well to dissolve. When reconstituted as directed, the solution contains approximately 1 mg of remifentanil activity per 1 mL.
- Remifentanil hydrochloride for injection should be diluted to a recommended final concentration of 20, 25, 50, or 250 mcg/mL prior to administration (see Table 5). Remifentanil hydrochloride for injection should not be administered without dilution.
Table 5: Reconstitution and Dilution of Remifentanil hydrochloride for injection
Final Concentration
Amount of Remifentanil hydrochloride for injection in Each Vial
Final Volume After Reconstitution and Dilution
20 mcg/mL
1 mg
50 mL
2 mg
100 mL
5 mg
250 mL
25 mcg/mL
1 mg
40 mL
2 mg
80 mL
5 mg
200 mL
50 mcg/mL
1 mg
20 mL
2 mg
40 mL
5 mg
100 mL
250 mcg/mL
5 mg
20 mL
Continuous IV infusions of Remifentanil hydrochloride for injection should be administered only by an infusion device. Infusion rates of Remifentanil hydrochloride for injection can be individualized for each patient using Table 6:
Table 6: IV Infusion Rates of Remifentanil hydrochloride for injection (mL/kg/h)
Drug Delivery Rate (mcg/kg/min)
Infusion Delivery Rate (mL/kg/h)
20 mcg/mL
25 mcg/mL
50 mcg/mL
250 mcg/mL
0.0125
0.038
0.03
0.015
not recommended
0.025
0.075
0.06
0.03
not recommended
0.05
0.15
0.12
0.06
0.012
0.075
0.23
0.18
0.09
0.018
0.1
0.3
0.24
0.12
0.024
0.15
0.45
0.36
0.18
0.036
0.2
0.6
0.48
0.24
0.048
0.25
0.75
0.6
0.3
0.06
0.5
1.5
1.2
0.6
0.12
0.75
2.25
1.8
0.9
0.18
1.0
3.0
2.4
1.2
0.24
1.25
3.75
3.0
1.5
0.3
1.5
4.5
3.6
1.8
0.36
1.75
5.25
4.2
2.1
0.42
2.0
6.0
4.8
2.4
0.48
When Remifentanil hydrochloride for injection is used as an analgesic component of monitored analgesia care, a final concentration of 25 mcg/mL is recommended. When Remifentanil hydrochloride for injection is used for pediatric patients 1 year of age and older, a final concentration of 20 or 25 mcg/mL is recommended. Table 7 is a guideline for milliliter-per-hour delivery for a solution of 20 mcg/mL with an infusion device.
Table 7: IV Infusion Rates of Remifentanil hydrochloride for injection (mL/h) for a 20 mcg/mL Solution
Infusion Rate
(mcg/kg/min)
Patient Weight (kg)
5
10
20
30
40
50
60
0.0125
0.188
0.375
0.75
1.125
1.5
1.875
2.25
0.025
0.375
0.75
1.5
2.25
3.0
3.75
4.5
0.05
0.75
1.5
3.0
4.5
6.0
7.5
9.0
0.075
1.125
2.25
4.5
6.75
9.0
11.25
13.5
0.1
1.5
3.0
6.0
9.0
12.0
15.0
18.0
0.15
2.25
4.5
9.0
13.5
18.0
22.5
27.0
0.2
3.0
6.0
12.0
18.0
24.0
30.0
36.0
0.25
3.75
7.5
15.0
22.5
30.0
37.5
45.0
0.3
4.5
9.0
18.0
27.0
36.0
45.0
54.0
0.35
5.25
10.5
21.0
31.5
42.0
52.5
63.0
0.4
6.0
12.0
24.0
36.0
48.0
60.0
72.0
Table 8 is a guideline for milliliter-per-hour delivery for a solution of 25 mcg/mL with an infusion device.
Table 8: IV Infusion Rates of Remifentanil hydrochloride for injection (mL/h) for a 25 mcg/mL Solution
Infusion Rate (mcg/kg/min)
Patient Weight (kg)
10
20
30
40
50
60
70
80
90
100
0.0125
0.3
0.6
0.9
1.2
1.5
1.8
2.1
2.4
2.7
3.0
0.025
0.6
1.2
1.8
2.4
3.0
3.6
4.2
4.8
5.4
6.0
0.05
1.2
2.4
3.6
4.8
6.0
7.2
8.4
9.6
10.8
12.0
0.075
1.8
3.6
5.4
7.2
9.0
10.8
12.6
14.4
16.2
18.0
0.1
2.4
4.8
7.2
9.6
12.0
14.4
16.8
19.2
21.6
24.0
0.15
3.6
7.2
10.8
14.4
18.0
21.6
25.2
28.8
32.4
36.0
0.2
4.8
9.6
14.4
19.2
24.0
28.8
33.6
38.4
43.2
48.0
Table 9 is a guideline for milliliter-per-hour delivery for a solution of 50 mcg/mL with an infusion device.
Table 9: IV Infusion Rates of Remifentanil hydrochloride for injection (mL/h) for a 50 mcg/mL Solution
Infusion Rate (mcg/kg/min)
Patient Weight (kg)
30
40
50
60
70
80
90
100
0.025
2.1
2.4
2.7
3.0
0.05
2.4
3.0
3.6
4.2
4.8
5.4
6.0
0.075
2.7
3.6
4.5
5.4
6.3
7.2
8.1
9.0
0.1
3.6
4.8
6.0
7.2
8.4
9.6
10.8
12.0
0.15
5.4
7.2
9.0
10.8
12.6
14.4
16.2
18.0
0.2
7.2
9.6
12.0
14.4
16.8
19.2
21.6
24.0
0.25
9.0
12.0
15.0
18.0
21.0
24.0
27.0
30.0
0.5
18.0
24.0
30.0
36.0
42.0
48.0
54.0
60.0
0.75
27.0
36.0
45.0
54.0
63.0
72.0
81.0
90.0
1.0
36.0
48.0
60.0
72.0
84.0
96.0
108.0
120.0
1.25
45.0
60.0
75.0
90.0
105.0
120.0
135.0
150.0
1.5
54.0
72.0
90.0
108.0
126.0
144.0
162.0
180.0
1.75
63.0
84.0
105.0
126.0
147.0
168.0
189.0
210.0
2.0
72.0
96.0
120.0
144.0
168.0
192.0
216.0
240.0
Table 10 is a guideline for milliliter-per-hour delivery for a solution of 250 mcg/mL with an infusion device.
Table 10: IV Infusion Rates of Remifentanil hydrochloride for injection (mL/h) for a 250 mcg/mL Solution
Infusion Rate (mcg/kg/min)
Patient Weight (kg)
30
40
50
60
70
80
90
100
0.1
0.72
0.96
1.20
1.44
1.68
1.92
2.16
2.40
0.15
1.08
1.44
1.80
2.16
2.52
2.88
3.24
3.60
0.2
1.44
1.92
2.40
2.88
3.36
3.84
4.32
4.80
0.25
1.80
2.40
3.00
3.60
4.20
4.80
5.40
6.00
0.5
3.60
4.80
6.00
7.20
8.40
9.60
10.80
12.00
0.75
5.40
7.20
9.00
10.80
12.60
14.40
16.20
18.00
1.0
7.20
9.60
12.00
14.40
16.80
19.20
21.60
24.00
1.25
9.00
12.00
15.00
18.00
21.00
24.00
27.00
30.00
1.5
10.80
14.40
18.00
21.60
25.20
28.80
32.40
36.00
1.75
12.60
16.80
21.00
25.20
29.40
33.60
37.80
42.00
2.0
14.40
19.20
24.00
28.80
33.60
38.40
43.20
48.00
Close2.12 Compatibility and Stability
Reconstitution and Dilution Prior to Administration
Remifentanil hydrochloride for injection is stable for 24 hours at room temperature after reconstitution and further dilution to concentrations of 20 to 250 mcg/mL with the IV fluids listed below.
Sterile Water for Injection, USP
5% Dextrose Injection, USP
5% Dextrose and 0.9% Sodium Chloride Injection, USP
0.9% Sodium Chloride Injection, USP
0.45% Sodium Chloride Injection, USP
Lactated Ringer's and 5% Dextrose Injection, USP
Remifentanil hydrochloride for injection is stable for 4 hours at room temperature after reconstitution and further dilution to concentrations of 20 to 250 mcg/mL with Lactated Ringer's Injection, USP.
Remifentanil hydrochloride for injection has been shown to be compatible with these IV fluids when coadministered into a running IV administration set.
Compatibility with Other Therapeutic Agents
Remifentanil hydrochloride for injection has been shown to be compatible with Diprivan® (propofol) Injection when coadministered into a running IV administration set. The compatibility of Remifentanil hydrochloride for injection with other therapeutic agents has not been evaluated.
Incompatibilities
Nonspecific esterases in blood products may lead to the hydrolysis of remifentanil to its carboxylic acid metabolite. Therefore, administration of Remifentanil hydrochloride for injection into the same IV tubing with blood is not recommended.
Note: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Product should be a clear, colorless liquid after reconstitution and free of visible particulate matter.
Remifentanil hydrochloride for injection does not contain any antimicrobial preservative and thus care must be taken to assure the sterility of prepared solutions.
-
3 DOSAGE FORMS AND STRENGTHSFor injection: 1 mg, 2 mg, and 5 mg: 3 mL Vial - 1 mg lyophilized powder - 5 mL Vial - 2 mg lyophilized powder - 10 mL Vial - 5 mg lyophilized powder
For injection: 1 mg, 2 mg, and 5 mg:
Close3 mL Vial
1 mg lyophilized powder
5 mL Vial
2 mg lyophilized powder
10 mL Vial
5 mg lyophilized powder
-
4 CONTRAINDICATIONSRemifentanil hydrochloride for injection is contraindicated: For epidural or intrathecal administration due to the presence of glycine in the formulation [see Nonclinical Toxicology (13)]. In ...
-
5 WARNINGS AND PRECAUTIONS5.1 Addiction, Abuse and Misuse - Remifentanil hydrochloride for injection contains remifentanil, a Schedule II controlled substance. As an opioid, Remifentanil hydrochloride for injection ...
5.1 Addiction, Abuse and Misuse
Remifentanil hydrochloride for injection contains remifentanil, a Schedule II controlled substance. As an opioid, Remifentanil hydrochloride for injection exposes users to the risks of addiction, abuse, and misuse [see Drug Abuse and Dependence (9)].
Opioids are sought for nonmedical use and are subject to diversion from legitimate prescribed use. Consider these risks when handling Remifentanil hydrochloride for injection. Strategies to reduce these risks include proper product storage and control practices for a C-II drug. Contact local state professional licensing board or state-controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
5.2 Life-Threatening Respiratory Depression
Serious, life-threatening, or fatal respiratory depression has been reported with the use of opioids, even when used as recommended. Respiratory depression, if not immediately recognized and treated, may lead to respiratory arrest and death.
Remifentanil hydrochloride for injection should be administered only by persons specifically trained in the use of anesthetic drugs and the management of the respiratory effects of potent opioids, including respiration and cardiac resuscitation of patients in the age group being treated. Such training must include the establishment and maintenance of a patent airway and assisted ventilation. Resuscitative and intubation equipment, oxygen, and opioid antagonists must be readily available.
Respiratory depression in spontaneously breathing patients is generally managed by decreasing the rate of the infusion of Remifentanil hydrochloride for injection by 50% or by temporarily discontinuing the infusion [see Overdosage (10)].
Carbon dioxide (CO2) retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids. While serious, life-threatening, or fatal respiratory depression can occur at any time during the use of Remifentanil hydrochloride for injection, the risk is greatest during the initiation of therapy or following a dosage increase. Monitor patients closely for respiratory depression, especially when initiating therapy with and following dosage increases of Remifentanil hydrochloride for injection.
Remifentanil hydrochloride for injection should not be used in diagnostic or therapeutic procedures outside the monitored anesthesia care setting. Patients receiving monitored anesthesia care should be continuously monitored by persons not involved in the conduct of the surgical or diagnostic procedure. Oxygen saturation should be monitored on a continuous basis.
Patients with significant chronic obstructive pulmonary disease or cor pulmonale, and those with a substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression are at increased risk of decreased respiratory drive including apnea, even at recommended dosages of Remifentanil hydrochloride for injection. Elderly, cachectic, or debilitated patients may have altered pharmacokinetics or altered clearance compared to younger, healthier patients resulting in greater risk for respiratory depression. Monitor such patients closely including vital signs, particularly when initiating and titrating Remifentanil hydrochloride for injection and when Remifentanil hydrochloride for injection is given concomitantly with other drugs that depress respiration. To reduce the risk of respiratory depression, proper dosing and titration of Remifentanil hydrochloride for injection are essential [see Dosage and Administration (2.11)].
5.3 Risks from Use as Postoperative Analgesia with Concomitant Benzodiazepines or Other CNS Depressants
When benzodiazepines or other CNS depressants are used with Remifentanil hydrochloride for injection, pulmonary arterial pressure may be decreased. This fact should be considered by those who conduct diagnostic and surgical procedures where interpretation of pulmonary arterial pressure measurements might determine final management of the patient. When high dose or anesthetic dosages of Remifentanil hydrochloride for injection are employed, even relatively small dosages of diazepam may cause cardiovascular depression.
When Remifentanil hydrochloride for injection is used with CNS depressants, hypotension can occur. If it occurs, consider the possibility of hypovolemia and manage with appropriate parenteral fluid therapy. When operative conditions permit, consider repositioning the patient to improve venous return to the heart. Exercise care in moving and repositioning of patients because of the possibility of orthostatic hypotension. If volume expansion with fluids plus other countermeasures do not correct hypotension, consider administration of pressor agents other than epinephrine. Epinephrine may paradoxically decrease blood pressure in patients treated with a neuroleptic that blocks alpha adrenergic activity.
Hypotension, profound sedation, respiratory depression, coma, and death may result from the concomitant use of Remifentanil hydrochloride for injection with benzodiazepines and/or other CNS depressants, including alcohol (e.g., non-benzodiazepine sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids). Patients should be advised to avoid alcohol for 24 hours after surgery [see Drug Interactions (7)].
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioid analgesics alone. Because of similar pharmacological properties, it is reasonable to expect similar risk with the concomitant use of other CNS depressant drugs with opioid analgesics [see Drug Interactions (7)].
If the decision is made to manage postoperative pain with Remifentanil hydrochloride for injection concomitantly with a benzodiazepine or other CNS depressant, start dosing with the lowest effective dosage and titrate based on clinical response. Monitor patients closely for signs and symptoms of respiratory depression, sedation, and hypotension. Fluids or other measures to counter hypotension should be available [see Drug Interactions (7)].5.4 Opioid-Induced Hyperalgesia and Allodynia
Opioid-Induced Hyperalgesia (OIH) occurs when an opioid analgesic paradoxically causes an increase in pain, or an increase in sensitivity to pain. This condition differs from tolerance, which is the need for increasing doses of opioids to maintain a defined effect [see Dependence (9.3)]. Symptoms of OIH include (but may not be limited to) increased levels of pain upon opioid dosage increase, decreased levels of pain upon opioid dosage decrease, or pain from ordinarily non-painful stimuli (allodynia). These symptoms may suggest OIH only if there is no evidence of underlying disease progression, opioid tolerance, opioid withdrawal, or addictive behavior.
Cases of OIH have been reported, both with short-term and longer-term use of opioid analgesics. Though the mechanism of OIH is not fully understood, multiple biochemical pathways have been implicated. Medical literature suggests a strong biologic plausibility between opioid analgesics and OIH and allodynia. If a patient is suspected to be experiencing OIH, carefully consider appropriately decreasing the dose of the current opioid analgesic or opioid rotation (safely switching the patient to a different opioid moiety) [see Dosage and Administration (2), Warnings and Precautions (5.2)].5.5 Serotonin Syndrome with Concomitant Use of Serotonergic Drugs
Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of Remifentanil hydrochloride for injection with serotonergic drugs. Serotonergic drugs include selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, drugs that affect the serotonergic neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), certain muscle relaxants (i.e., cyclobenzaprine, metaxalone), and drugs that impair metabolism of serotonin (including MAO inhibitors, both those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue) [see Drug Interactions (7)]. This may occur within the recommended dosage range.
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination, rigidity), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea) and can be fatal. The onset of symptoms generally occurs within several hours to a few days of concomitant use, but may occur later than that. Discontinue Remifentanil hydrochloride for injection if serotonin syndrome is suspected.
5.6 Administration
Continuous infusions of Remifentanil hydrochloride for injection should be administered only by an infusion device. IV bolus administration of Remifentanil hydrochloride for injection should be used only during the maintenance of general anesthesia. In nonintubated patients, single doses of Remifentanil hydrochloride for injection should be administered over 30 to 60 seconds.
Interruption of an infusion of Remifentanil hydrochloride for injection will result in rapid offset of effect. Rapid clearance and lack of drug accumulation result in rapid dissipation of respiratory depressant and analgesic effects upon discontinuation of Remifentanil hydrochloride for injection at recommended doses. Discontinuation of an infusion of Remifentanil hydrochloride for injection should be preceded by the establishment of adequate postoperative analgesia.
Injections of Remifentanil hydrochloride for injection should be made into IV tubing at or close to the venous cannula. Upon discontinuation of Remifentanil hydrochloride for injection, the IV tubing should be cleared to prevent the inadvertent administration of Remifentanil hydrochloride for injection at a later point in time. Failure to adequately clear the IV tubing to remove residual Remifentanil hydrochloride for injection has been associated with the appearance of respiratory depression, apnea, and muscle rigidity upon the administration of additional fluids or medications through the same IV tubing.
5.7 Skeletal Muscle Rigidity
Skeletal muscle rigidity can be caused by Remifentanil hydrochloride for injection and is related to the dose and speed of administration. Remifentanil hydrochloride for injection may cause chest wall rigidity (inability to ventilate) after single doses of > 1 mcg/kg administered over 30 to 60 seconds, or after infusion rates > 0.1 mcg/kg/min. Single doses < 1 mcg/kg may cause chest wall rigidity when given concurrently with a continuous infusion of Remifentanil hydrochloride for injection.
Muscle rigidity induced by Remifentanil hydrochloride for injection should be managed in the context of the patient's clinical condition. Muscle rigidity occurring during the induction of anesthesia should be treated by the administration of a neuromuscular blocking agent and the concurrent induction medications and can be treated by decreasing the rate or discontinuing the infusion of Remifentanil hydrochloride for injection or by administering a neuromuscular blocking agent. The neuromuscular blocking agents used should be compatible with the patient's cardiovascular status.
Muscle rigidity seen during the use of Remifentanil hydrochloride for injection in spontaneously breathing patients may be treated by stopping or decreasing the rate of administration of Remifentanil hydrochloride for injection. Resolution of muscle rigidity after discontinuing the infusion of Remifentanil hydrochloride for injection occurs within minutes. In the case of life-threatening muscle rigidity, a rapid onset neuromuscular blocker or naloxone may be administered.
5.8 Potential Inactivation by Nonspecific Esterases in Blood Products
Remifentanil hydrochloride for injection should not be administered into the same IV tubing with blood due to potential inactivation by nonspecific esterases in blood products.
5.9 Bradycardia
Bradycardia has been reported with Remifentanil hydrochloride for injection and is responsive to ephedrine or anticholinergic drugs, such as atropine and glycopyrrolate.
5.10 Hypotension
Hypotension has been reported with Remifentanil hydrochloride for injection and is responsive to decreases in the administration of Remifentanil hydrochloride for injection or to IV fluids or catecholamine (ephedrine, epinephrine, norepinephrine, etc.) administration.
5.11 Intraoperative Awareness
Intraoperative awareness has been reported in patients under 55 years of age when Remifentanil hydrochloride for injection has been administered with propofol infusion rates of ≤ 75 mcg/kg/min.
5.12 Risks of Use in Spontaneously Breathing Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness
In patients who may be susceptible to the intracranial effects of CO2 retention (e.g., those with evidence of increased intracranial pressure or brain tumors), Remifentanil hydrochloride for injection may reduce respiratory drive, and the resultant CO2 retention can further increase intracranial pressure in spontaneously breathing patients. Monitor such patients for signs of sedation and respiratory depression, particularly when initiating therapy with Remifentanil hydrochloride for injection.
Opioids may also obscure the clinical course in a patient with a head injury.
5.13 Risks of Use in Patients with Biliary Tract Disease
The remifentanil in Remifentanil hydrochloride for injection may cause spasm of the sphincter of Oddi. Opioids may cause increases in serum amylase. Monitor patients with biliary tract disease, including acute pancreatitis, for worsening symptoms.
5.14 Increased Risk of Seizures in Patients with Seizure Disorders
The remifentanil in Remifentanil hydrochloride for injection may increase the frequency of seizures in patients with seizure disorders, and may increase the risk of seizures occurring in other clinical settings associated with seizures. Monitor patients with a history of seizure disorders for worsened seizure control during Remifentanil hydrochloride for injection therapy.
Close5.15 Rapid Offset of Action
Analgesic activity will subside within 5 to 10 minutes after discontinuation of administration of Remifentanil hydrochloride for injection. However, respiratory depression may continue in some patients for up to 30 minutes after termination of infusion due to residual effects of concomitant anesthetics. Standard monitoring should be maintained in the postoperative period to ensure adequate recovery without stimulation. For patients undergoing surgical procedures where postoperative pain is generally anticipated, other analgesics should be administered prior to the discontinuation of Remifentanil hydrochloride for injection.
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described, or described in greater detail, in other sections: Addiction, Abuse, and Misuse [see Warnings and Precautions (5.1)] Life-Threatening ...
The following serious adverse reactions are described, or described in greater detail, in other sections:
- Addiction, Abuse, and Misuse [see Warnings and Precautions (5.1)]
- Life-Threatening Respiratory Depression [see Warnings and Precautions (5.2)]
- Interactions with Benzodiazepines or other CNS Depressants [see Warnings and Precautions (5.3)]
- Opioid-Induced Hyperalgesia and Allodynia [see Warnings and Precautions (5.4)]
- Serotonin Syndrome [see Warnings and Precautions (5.5)]
- Skeletal Muscle Rigidity [see Warnings and Precautions (5.7)]
- Bradycardia [see Warnings and Precautions (5.9)]
- Hypotension [see Warnings and Precautions (5.10)]
- Biliary Tract Disease [see Warnings and Precautions (5.13)]
- Seizures [see Warnings and Precautions (5.14)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse event information is derived from controlled clinical studies that were conducted in a variety of surgical procedures of varying duration, using a variety of premedications and other anesthetics, and in patient populations with diverse characteristics including underlying disease.
Adults
Approximately 2,770 adult patients were exposed to Remifentanil hydrochloride for injection in controlled clinical studies. The frequencies of adverse events during general anesthesia with the recommended doses of Remifentanil hydrochloride for injection are given in Table 11. Each patient was counted once for each type of adverse event.
Table 11: Adverse Events Reported in ≥ 1% of Adult Patients in General Anesthesia Studiesa at the Recommended Dosesb of Remifentanil hydrochloride for injection
Adverse Event
Induction/Maintenance
Postoperative Analgesia
After Discontinuation
Remifentanil hydrochloride for injection
(n = 921)
Alfentanil/Fentanyl (n = 466)
Remifentanil hydrochloride for injection
(n = 281)
Morphine (n = 98)
Remifentanil hydrochloride for injection
(n = 929)
Alfentanil/Fentanyl (n = 466)
Nausea
8 (< 1%)
0
61 (22%)
15 (15%)
339 (36%)
202 (43%)
Hypotension
178 (19%)
30 (6%)
0
0
16 (2%)
9 (2%)
Vomiting
4 (< 1%)
1 (< 1%)
22 (8%)
5 (5%)
150 (16%)
91 (20%)
Muscle rigidity
98 (11%)c
37 (8%)
7 (2%)
0
2 (< 1%)
1 (< 1%)
Bradycardia
62 (7%)
24 (5%)
3 (1%)
3 (3%)
11 (1%)
6 (1%)
Shivering
3 (< 1%)
0
15 (5%)
9 (9%)
49 (5%)
10 (2%)
Fever
1 (< 1%)
0
2 (< 1%)
0
44 (5%)
9 (2%)
Dizziness
0
0
1 (< 1%)
0
27 (3%)
9 (2%)
Visual disturbance
0
0
0
0
24 (3%)
14 (3%)
Headache
0
0
1 (< 1%)
1 (1%)
21 (2%)
8 (2%)
Respiratory depression
1 (< 1%)
0
19 (7%)
4 (4%)
17 (2%)
20 (4%)
Apnea
0
1 (< 1%)
9 (3%)
2 (2%)
2 (< 1%)
1 (< 1%)
Pruritus
2 (< 1%)
0
7 (2%)
1 (1%)
22 (2%)
7 (2%)
Tachycardia
6 (< 1%)
7 (2%)
0
0
10 (1%)
8 (2%)
Postoperative pain
0
0
7 (2%)
0
4 (< 1%)
5 (1%)
Hypertension
10 (1%)
7 (2%)
5 (2%)
3 (3%)
12 (1%)
8 (2%)
Agitation
2 (< 1%)
0
3 (1%)
1 (1%)
6 (< 1%)
1 (< 1%)
Hypoxia
0
0
1 (< 1%)
0
10 (1%)
7 (2%)
a Does not include adverse events from cardiac studies or the neonatal study. See Tables 14, 15, and 16 for cardiac information.
b See Table 1 for recommended doses. Not all doses of Remifentanil hydrochloride for injection were equipotent to the comparator opioid. Administration of Remifentanil hydrochloride for injection in excess of the recommended dose (i.e., doses > 1 and up to 20 mcg/kg) resulted in a higher incidence of some adverse events: muscle rigidity (37%), bradycardia (12%), hypertension (4%), and tachycardia (4%).
c Included in the muscle rigidity incidence is chest wall rigidity (5%). The overall muscle rigidity incidence is < 1% when remifentanil is administered concurrently or after a hypnotic induction agent.
In the elderly population (> 65 years), the incidence of hypotension is higher, whereas the incidence of nausea and vomiting is lower.
Table 12: Incidence (%) of Most Common Adverse Events by Gender in General Anesthesia Studiesa at the Recommended Dosesb of Remifentanil hydrochloride for injection
Adverse Event
n
Induction Maintenance
Postoperative Analgesia
After Discontinuation
Remifentanil hydrochloride for injection
Alfentanil/
Fentanyl
Remifentanil hydrochloride for injection
Morphine
Remifentanil hydrochloride for injection
Alfentanil/
Fentanyl
Male 326
Female 595
Male 183
Female 283
Male 85
Female 196
Male 36
Female 62
Male 332
Female 597
Male 183
Female 283
Nausea
2%
< 1%
0
0
12%
26%
8%
19%
22%
45%
30%
52%
Hypotension
29%
14%
7%
6%
0
0
0
0
2%
2%
2%
2%
Vomiting
< 1%
< 1%
0
< 1%
4%
10%
0
8%
5%
22%
8%
27%
Muscle rigidity
17%
7%
14%
4%
6%
1%
0
0
< 1%
< 1%
0
< 1%
a Does not include adverse events from cardiac studies or the neonatal study.
b See Table 1 for recommended doses. Not all doses of Remifentanil hydrochloride for injection were equipotent to the comparator opioid.
The frequencies of adverse events from the clinical studies at the recommended doses of Remifentanil hydrochloride for injection in monitored anesthesia care are given in Table 13.
Table 13: Adverse Events Reported in ≥ 1% of Adult Patients in Monitored Anesthesia Care Studies at the Recommended Dosesa of Remifentanil hydrochloride for injection
Adverse Event
Remifentanil hydrochloride for injection
(n = 159)
Remifentanil hydrochloride for injection + 2 mg Midazolamb
(n = 103)
Propofol (0.5 mg/kg then 50 mcg/kg/min)
(n = 63)
Nausea
70 (44%)
19 (18%)
20 (32%)
Vomiting
35 (22%)
5 (5%)
13 (21%)
Pruritus
28 (18%)
16 (16%)
0
Headache
28 (18%)
12 (12%)
6 (10%)
Sweating
10 (6%)
0
1 (2%)
Shivering
8 (5%)
1 (< 1%)
1 (2%)
Dizziness
8 (5%)
5 (5%)
1 (2%)
Hypotension
7 (4%)
0
6 (10%)
Bradycardia
6 (4%)
0
7 (11%)
Respiratory depression
4 (3%)
1 (< 1%)a
0
Muscle rigidity
4 (3%)
0
1 (2%)
Chills
2 (1%)
0
2 (3%)
Flushing
2 (1%)
0
0
Warm sensation
2 (1%)
0
0
Pain at study IV site
2 (1%)
0
11 (17%)
a See Table 3 for recommended doses. Administration of Remifentanil hydrochloride for injection in excess of the recommended infusion rate (i.e., starting doses > 0.1 mcg/kg/min) resulted in a higher incidence of some adverse events: nausea (60%), apnea (8%), and muscle rigidity (5%).
b With higher midazolam doses, higher incidences of respiratory depression and apnea were observed.
Other Adverse Events in Adult Patients
The frequencies of less commonly reported adverse clinical events from all controlled general anesthesia and monitored anesthesia care studies are presented below.
Event frequencies are calculated as the number of patients who were administered Remifentanil hydrochloride for injection and reported an event divided by the total number of patients exposed to Remifentanil hydrochloride for injection in all controlled studies including cardiac dose-ranging and neurosurgery studies (n = 1,883 general anesthesia, n = 609 monitored anesthesia care).
Incidence Less than 1%
Digestive: constipation, abdominal discomfort, xerostomia, gastro-esophageal reflux, dysphagia, diarrhea, ileus.
Cardiovascular: various atrial and ventricular arrhythmias, heart block, ECG change consistent with myocardial ischemia, elevated CPK-MB level, syncope.
Musculoskeletal: muscle stiffness, musculoskeletal chest pain.
Respiratory: cough, dyspnea, bronchospasm, laryngospasm, rhonchi, stridor, nasal congestion, pharyngitis, pleural effusion, hiccup(s), pulmonary edema, rales, bronchitis, rhinorrhea.
Nervous: anxiety, involuntary movement, prolonged emergence from anesthesia, confusion, awareness under anesthesia without pain, rapid awakening from anesthesia, tremors, disorientation, dysphoria, nightmare(s), hallucinations, paresthesia, nystagmus, twitch, seizure, amnesia.
Body as a Whole: decreased body temperature, anaphylactic reaction, delayed recovery from neuromuscular block.
Skin: rash, urticaria.
Urogenital: urine retention, oliguria, dysuria, urine incontinence.
Infusion Site Reaction: erythema, pruritus, rash.
Metabolic and Nutrition: abnormal liver function, hyperglycemia, electrolyte disorders, increased CPK level.
Hematologic and Lymphatic: anemia, lymphopenia, leukocytosis, thrombocytopenia.
The frequencies of adverse events from the clinical studies at the recommended doses of Remifentanil hydrochloride for injection in cardiac surgery are given in Tables 14, 15, and 16. These tables represent adverse events collected during discrete phases of cardiac surgery. Any event should be viewed as temporally associated with drug administration and the phase indicated should not be perceived as the only time the event might occur.
Table 14: Adverse Events Reported in ≥ 1% of Patients in the Induction/Intubation and Maintenance Phases of Cardiac Surgery Studies at the Recommended Dosesa of Remifentanil hydrochloride for injection
Induction/Intubation
Maintenance
Adverse Event
Remifentanil hydrochloride for injection
(n = 227)
Fentanyl
(n = 176)
Sufentanil
(n = 41)
Remifentanil hydrochloride for injection
(n = 227)
Fentanyl
(n = 176)
Sufentanil
(n = 41)
Hypotension
18 (8%)
6 (3%)
7 (17%)
26 (11%)
6 (3%)
1 (2%)
Bradycardia
9 (4%)
5 (3%)
0
3 (1%)
1 (< 1%)
1 (2%)
Hypertension
3 (1%)
2 (1%)
2 (5%)
8 (4%)
6 (3%)
1 (2%)
Constipation
9 (4%)
1 (< 1%)
3 (7%)
0
0
1 (2%)
Muscle rigidity
2 (< 1%)
2 (1%)
0
5 (2%)
8 (5%)
0
Premature ventricular beats
1 (< 1%)
0
0
3 (1%)
1 (< 1%)
0
Myocardial ischemia
0
0
0
7 (3%)
8 (5%)
1 (2%)
Atrial fibrillation
0
0
0
7 (3%)
3 (2%)
1 (2%)
Decreased cardiac output
0
0
0
5 (2%)
1 (< 1%)
1 (2%)
Tachycardia
0
1 (< 1%)
0
4 (2%)
2 (1%)
0
Coagulation disorder
0
0
0
4 (2%)
0
1 (2%)
Arrhythmia
0
0
0
3 (1%)
0
0
Ventricular fibrillation
0
0
0
3 (1%)
1 (< 1%)
1 (2%)
Postoperative complication
0
0
0
3 (1%)
0
0
Third degree heart block
0
0
0
2 (< 1%)
0
1 (2%)
Hemorrhage
0
0
0
2 (< 1%)
0
1 (2%)
Perioperative complication
0
0
0
2 (< 1%)
1 (< 1%)
1 (2%)
Involuntary movement(s)
0
0
0
2 (< 1%)
3 (2%)
0
Thrombocytopenia
0
0
1 (2%)
0
0
0
Oliguria
0
0
0
0
3 (2%)
0
Anemia
0
0
0
2 (< 1%)
2 (1%)
0
aSee Table 4 for recommended doses.
Table 15: Adverse Events Reported in ≥ 1% of Patients in the ICU Phase of Cardiac Surgery Studies at the Recommended Dosesa of Remifentanil hydrochloride for injection
Adverse Event
Remifentanil hydrochloride for injection
n = 227
Fentanyl
n = 176
Sufentanil
n = 41
Hypertension
14 (6%)
8 (5%)
2 (5%)
Hypotension
12 (5%)
3 (2%)
1 (2%)
Tachycardia
9 (4%)
5 (3%)
0
Shivering
8 (4%)
3 (2%)
1 (2%)
Nausea
8 (4%)
3 (2%)
0
Hemorrhage
4 (2%)
1 (< 1%)
1 (2%)
Postoperative complication
4 (2%)
5 (3%)
2 (5%)
Agitation
4 (2%)
1 (< 1%)
1 (2%)
Ache
4 (2%)
0
0
Decreased cardiac output
3 (1%)
0
0
Arrhythmia
3 (1%)
0
0
Muscle rigidity
2 (< 1%)
1 (< 1%)
2 (5%)
Bradycardia
2 (< 1%)
2 (1%)
0
Vomiting
1 (< 1%)
2 (1%)
0
Premature ventricular beats
1 (< 1%)
2 (1%)
0
Anemia
0
3 (2%)
0
Somnolence
0
0
1 (2%)
Fever
0
2 (1%)
0
a See Table 4 for recommended doses.
Table 16: Adverse Events Reported in ≥ 1% of Patients in the Post-Study Drug Phase of Cardiac Surgery Studies at the Recommended Dosesa of Remifentanil hydrochloride for injection
Adverse Event
Remifentanil hydrochloride for injection
n = 227
Fentanyl
n = 176
Sufentanil
n = 41
Nausea
90 (40%)
63 (36%)
16 (39%)
Vomiting
33 (15%)
26 (15%)
3 (7%)
Fever
30 (13%)
15 (9%)
0
Atrial fibrillation
27 (12%)
33 (19%)
4 (10%)
Constipation
20 (9%)
35 (20%)
3 (7%)
Pleural effusion
11 (5%)
2 (1%)
2 (5%)
Hypotension
8 (4%)
8 (5%)
1 (2%)
Tachycardia
9 (4%)
15 (9%)
0
Postoperative complication
10 (4%)
6 (3%)
2 (5%)
Oliguria
7 (3%)
7 (4%)
1 (2%)
Confusion
7 (3%)
10 (6%)
5 (12%)
Ache
6 (3%)
2 (1%)
0
Anxiety
6 (3%)
6 (3%)
0
Headache
6 (3%)
2 (1%)
0
Perioperative complication
5 (2%)
7 (4%)
1 (2%)
Anemia
5 (2%)
5 (3%)
1 (2%)
Agitation
5 (2%)
3 (2%)
1 (2%)
Diarrhea
5 (2%)
1 (< 1%)
1 (2%)
Edema
4 (2%)
6 (3%)
0
Dizziness
4 (2%)
3 (2%)
1 (2%)
Postoperative infection
5 (2%)
7 (4%)
0
Hypoxia
4 (2%)
5 (3%)
0
Apnea
4 (2%)
1 (< 1%)
1 (2%)
Hypertension
3 (1%)
3 (2%)
0
Shivering
3 (1%)
1 (< 1%)
0
Heartburn
3 (1%)
3 (2%)
0
Atrial flutter
3 (1%)
1 (< 1%)
0
Arrhythmia
3 (1%)
5 (3%)
0
Hallucinations
3 (1%)
3 (2%)
0
Pneumonia
3 (1%)
3 (2%)
1 (2%)
Pharyngitis
3 (1%)
1 (< 1%)
1 (2%)
Decreased mental acuity
3 (1%)
1 (< 1%)
0
Dyspnea
3 (1%)
1 (< 1%)
0
Cough
3 (1%)
0
0
Decreased cardiac output
1 (< 1%)
0
3 (7%)
Renal insufficiency
1 (< 1%)
5 (3%)
0
Bradycardia
1 (< 1%)
1 (< 1%)
1 (2%)
Urine retention
2 (< 1%)
3 (2%)
0
Cerebral infarction
2 (< 1%)
2 (1%)
1 (2%)
Premature ventricular beats
2 (< 1%)
3 (2%)
0
Cerebral ischemia
1 (< 1%)
1 (< 1%)
1 (2%)
Paresthesia
2 (< 1%)
2 (1%)
0
Seizure
2 (< 1%)
1 (< 1%)
1 (2%)
Sleep disorder
1 (< 1%)
1 (< 1%)
1 (2%)
Bronchospasm
1 (< 1%)
6 (3%)
0
Atelectasis
2 (< 1%)
3 (2%)
0
Respiratory depression
2 (< 1%)
3 (2%)
0
Pulmonary edema
1 (< 1%)
2 (1%)
0
Respiratory distress
2 (< 1%)
0
1 (2%)
Hyperkalemia
2 (< 1%)
3 (2%)
0
Electrolyte disorder
0
3 (2%)
0
Chest congestion
0
3 (2%)
0
Hemoptysis
0
2 (1%)
0
Facial ptosis
0
2 (1%)
0
Hemorrhage
0
2 (1%)
0
Hematuria
0
1 (< 1%)
1 (2%)
Visual disturbance(s)
0
1 (< 1%)
1 (2%)
Hypokalemia
0
2 (1%)
0
Exacerbation of renal failure
0
0
1 (2%)
Blood in stool
0
0
1 (2%)
First degree heart block
0
0
1 (2%)
Pericarditis
0
0
1 (2%)
a See Table 4 for recommended doses.
Pediatrics
Remifentanil hydrochloride for injection has been studied in 342 pediatric patients in controlled clinical studies for maintenance of general anesthesia. In the pediatric population (birth to 12 years), the most commonly reported events were nausea, vomiting, and shivering.
The frequencies of adverse events during general anesthesia with the recommended doses of Remifentanil hydrochloride for injection are given in Table 17. Each patient was counted once for each type of adverse event.
There were no adverse events ≥ 1% for any treatment group during the maintenance period in the pediatric patient general anesthesia studies.
Table 17: Adverse Events Reported in ≥ 1% of Pediatric Patients Receiving Remifentanil hydrochloride for injection in General Anesthesia Studies at the Recommended Dosesa of Remifentanil hydrochloride for injection
Recovery
Follow-upb
Adverse Event
Remifentanil hydrochloride for injection
(n = 342)
Fentanyl (n = 103)
Bupivacaine (n = 86)
Remifentanil hydrochloride for injection (n = 342)
Fentanyl (n = 103)
Bupivacaine (n = 86)
Vomiting
40 (12%)
9 (9%)
10 (12%)
56 (16%)
8 (8%)
12 (14%)
Nausea
23 (8%)
7 (7%)
1 (1%)
17 (6%)
6 (6%)
5 (6%)
Shivering
9 (3%)
0
0
0
0
0
Rhonchi
8 (3%)
2 (2%)
0
0
0
0
Postoperative complication
5 (2%)
2 (2%)
0
4 (1%)
0
0
Stridor
4 (1%)
2 (2%)
0
0
0
0
Cough
4 (1%)
1 (< 1%)
0
0
0
0
a See Table 2 for recommended doses.
b In subjects receiving halothane (n = 22), 10 (45%) experienced vomiting.
Close6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of remifentanil. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular: Asystole
Serotonin syndrome: Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of opioids with serotonergic drugs.
Anaphylaxis: Anaphylaxis has been reported with ingredients contained in Remifentanil hydrochloride for injection.
Hyperalgesia and Allodynia: Cases of hyperalgesia and allodynia have been reported with opioid therapy of any duration [see Warnings and Precautions (5.4)].
Hypoglycemia: Cases of hypoglycemia have been reported in patients taking opioids. Most reports were in patients with at least one predisposing risk factor (e.g., diabetes). -
7 DRUG INTERACTIONSTable 18 includes clinically significant drug interactions with Remifentanil hydrochloride for injection. Table 18: Clinically Significant Drug Interactions with Remifentanil hydrochloride ...
Table 18 includes clinically significant drug interactions with Remifentanil hydrochloride for injection.
Table 18: Clinically Significant Drug Interactions with Remifentanil hydrochloride for injection
CloseBenzodiazepines and other Central Nervous System (CNS) Depressants
Clinical Impact:
Due to additive pharmacologic effect, the concomitant use of benzodiazepines or other CNS depressants including alcohol, increases the risk of hypotension, respiratory depression, profound sedation, coma, and death.
Intervention:
Limit dosages and durations to the minimum required. Follow patients closely for signs of respiratory depression and sedation. Patients should be advised to avoid alcohol for 24 hours after surgery [see Warnings and Precautions (5.3)].
Examples:
Benzodiazepines and other sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids, alcohol.
Serotonergic Drugs
Clinical Impact:
The concomitant use of opioids with other drugs that affect the serotonergic neurotransmitter system has resulted in serotonin syndrome [see Warnings and Precautions (5.5)].
Intervention:
If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment. Discontinue Remifentanil hydrochloride for injection if serotonin syndrome is suspected.
Examples:
Selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, drugs that effect the serotonin neurotransmitter system (e.g.,
mirtazapine, trazodone, tramadol), certain muscle relaxants (i.e., cyclobenzaprine, metaxalone), monoamine oxidase (MAO) inhibitors (those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue).
Monoamine Oxidase Inhibitors (MAOIs)
Clinical Impact:
MAOI interactions with opioids may manifest as serotonin syndrome [see Warnings and Precautions (5.5)] or opioid toxicity (e.g., respiratory depression, coma) [see Warnings and Precautions (5.2)].
If urgent use of Remifentanil hydrochloride for injection is necessary, use test doses and frequent titration of small doses while closely monitoring blood pressure and signs and symptoms of CNS and respiratory depression.
Intervention:
The use of Remifentanil hydrochloride for injection is not recommended for patients taking MAOIs or within 14 days of stopping such treatment.
Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics
Clinical Impact:
May reduce the analgesic effect of Remifentanil hydrochloride for injection and/or precipitate withdrawal symptoms.
Intervention:
If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment. Consider discontinuing Remifentanil hydrochloride for injection if patient is not responding appropriately to treatment and institute alternative analgesic treatment.
Examples:
butorphanol, nalbuphine, pentazocine, buprenorphine
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Use of opioid analgesics for an extended period of time during pregnancy may cause neonatal opioid withdrawal syndrome. Available data with remifentanil ...
8.1 Pregnancy
Risk Summary
Use of opioid analgesics for an extended period of time during pregnancy may cause neonatal opioid withdrawal syndrome. Available data with remifentanil hydrochloride in pregnant women are insufficient to inform a drug-associated risk for major birth defects and miscarriage. In animal reproduction studies, reduced fetal rat body weight and pup weights were reported at 2.2 times a human intravenous infusion of an induction dose of 1 mcg/kg with a maintenance dose of 2 mcg/kg/min for a surgical procedure lasting 3 hours. There were no malformations noted when remifentanil was administered via bolus injection to pregnant rats or rabbits during organogenesis at doses approximately 5 times and approximately equal, respectively, to a human intravenous infusion of an induction dose of 1 mcg/kg with a maintenance dose of 2 mcg/kg/min for a surgical procedure lasting 3 hours [see Data]. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Labor or Delivery
Opioids cross the placenta and may produce respiratory depression and psycho-physiologic effects in neonates. An opioid antagonist, such as naloxone, must be available for reversal of opioid-induced respiratory depression in the neonate. Remifentanil hydrochloride for injection is not recommended for use in pregnant women during or immediately prior to labor, when other analgesic techniques are more appropriate. Opioid analgesics, including Remifentanil hydrochloride for injection, can prolong labor through actions which temporarily reduce the strength, duration, and frequency of uterine contractions. However, this effect is not consistent and may be offset by an increased rate of cervical dilation, which tends to shorten labor. Monitor neonates exposed to opioid analgesics during labor for signs of excess sedation and respiratory depression.
Data
Human Data
In a human clinical trial, the average maternal remifentanil concentrations were approximately twice those seen in the fetus. In some cases, however, fetal concentrations were similar to those in the mother. The umbilical arteriovenous ratio of remifentanil concentrations was approximately 30% suggesting metabolism of remifentanil in the neonate.
Animal Data
Pregnant rats were treated from Gestation Day 6 to 15 with intravenous remifentanil doses of 0.5, 1.6, or 5 mg/kg/day (0.2, 0.7, or 2.2 times a human intravenous infusion of an induction dose of 1 mcg/kg with a maintenance dose of 2 mcg/kg/min based on body surface area for a surgical procedure lasting 3 hours based on body surface area, respectively). Reduced fetal weights were reported in the high dose group; however, no malformations were reported in surviving fetuses despite a non-dose dependent increase in maternal mortality.
Pregnant rabbits were treated from Gestation Day 6 to 18 with intravenous remifentanil doses of 0.1, 0.5, or 0.8 mg/kg/day (0.09, 0.4, or 0.7 times a human intravenous infusion of an induction dose of 1 mcg/kg with a maintenance dose of 2 mcg/kg/min based on body surface area for a surgical procedure lasting 3 hours based on body surface area, respectively). No malformations were reported in surviving fetuses despite clear maternal toxicity (decreased food consumption and body weights and increased mortality in all treatment groups).
Pregnant rats were treated from Gestation Day 6 to Lactation Day 21 with intravenous boluses of remifentanil 0.5, 1.6, or 5 mg/kg/day (0.2, 0.7, or 2.2 times a human intravenous infusion of an induction dose of 1 mcg/kg with a maintenance dose of 2 mcg/kg/min based on body surface area for a surgical procedure lasting 3 hours based on body surface area, respectively). Reduced birth weights were noted in the high-dose groups in the presence of maternal toxicity (increased mortality in all groups).
8.2 Lactation
Risk Summary
It is not known whether remifentanil is excreted in human milk. After receiving radioactive-labeled remifentanil, the radioactivity was present in the milk of lactating rats. Because fentanyl analogs are excreted in human milk, caution should be exercised when Remifentanil hydrochloride for injection is administered to a nursing woman.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Remifentanil hydrochloride for injection and any potential adverse effects on the breastfed infant from Remifentanil hydrochloride for injection or from the underlying maternal condition.
Clinical Considerations
Infants exposed to Remifentanil hydrochloride for injection through breast milk should be monitored for excess sedation and respiratory depression. Withdrawal symptoms can occur in breastfed infants when maternal administration of an opioid analgesic is stopped, or when breast-feeding is stopped.
8.4 Pediatric Use
The efficacy and safety of Remifentanil hydrochloride for injection as an analgesic agent for use in the maintenance of general anesthesia in outpatient and inpatient pediatric surgery have been established in controlled clinical studies in pediatric patients from birth to 12 years [see Clinical Studies (14.4)].
The initial maintenance infusion regimen of Remifentanil hydrochloride for injection evaluated in pediatric patients from birth to 2 months of age was 0.4 mcg/kg/min, the approved adult regimen for use with N2O. The clearance rate observed in neonates was highly variable and on average was 2 times higher than in the young healthy adult population. Therefore, while a starting infusion rate of 0.4 mcg/kg/min may be appropriate for some neonates, an increased infusion rate may be necessary to maintain adequate surgical anesthesia, and additional bolus doses may be required. The individual dose for each patient should be carefully titrated. [See Clinical Pharmacology: Specific Populations: Pediatric Population (12.3) and Dosage and Administration, Table 2 and Maintenance of Anesthesia (2.2).]
Remifentanil hydrochloride for injection has not been studied in pediatric patients for use as a postoperative analgesic or as an analgesic component of monitored anesthesia care.
8.5 Geriatric Use
Respiratory depression is the chief risk for elderly patients treated with opioids and has occurred after large initial doses were administered to patients who were not opioid-tolerant or when opioids were co-administered with other agents that depress respiration. Titrate the dosage of Remifentanil hydrochloride for injection slowly in geriatric patients and frequently reevaluate the patient for signs of central nervous system and respiratory depression [see Warnings and Precautions (5.2)]
Of the total number of subjects in clinical studies of Remifentanil hydrochloride for injection, 486 were 65 and over (age range 66 to 90 years). While the effective biological half-life of remifentanil is unchanged, elderly patients have been shown to be twice as sensitive as the younger population to the pharmacodynamic effects of remifentanil. The recommended starting dose of Remifentanil hydrochloride for injection should be decreased by 50% in patients over 65 years of age [see Clinical Pharmacology (12.3) and Dosage and Administration (2.2)]. Titrate the dosage of Remifentanil hydrochloride for injection slowly in geriatric patients. [See Warnings and Precautions (5.5).]The clearance of remifentanil is reduced (approximately 25%) in the elderly (> 65 years of age) compared to young adults (average 25 years of age). However, remifentanil blood concentrations fall as rapidly after termination of administration in the elderly as in young adults.
This drug is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.8.6 Use in Morbidly Obese Patients
As for all potent opioids, caution is required with use in morbidly obese patients because of alterations in cardiovascular and respiratory physiology [see Dosage and Administration (2.2)].
Close8.7 Long-Term Use in the ICU
No data are available on the long-term (longer than 16 hours) use of Remifentanil hydrochloride for injection as an analgesic in ICU patients.
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance - Remifentanil hydrochloride for injection contains remifentanil, a Schedule II controlled substance. 9.2 Abuse - Remifentanil hydrochloride for injection contains ...
9.1 Controlled Substance
Remifentanil hydrochloride for injection contains remifentanil, a Schedule II controlled substance.
9.2 Abuse
Remifentanil hydrochloride for injection contains remifentanil, a substance with high potential for misuse and abuse, which can lead to the development of substance use disorder, including addiction [see Warnings and Precautions (5.1)].
Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a healthcare provider or for whom it was not prescribed.
Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects.
Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence.
Misuse and abuse of Remifentanil hydrochloride for injection increases risk of overdosage, which may lead to central nervous system and respiratory depression, hypotension, seizures, and death. The risk is increased with concurrent abuse of Remifentanil hydrochloride for injection with alcohol and/or other CNS depressants. Abuse of and addiction to opioids in some individuals may not be accompanied by concurrent tolerance and symptoms of physical dependence. In addition, abuse of opioids can occur in the absence of addiction.
All patients treated with opioids require careful and frequent reevaluation for signs of misuse, abuse, and addiction, because use of opioid analgesic products carries the risk of addiction even under appropriate medical use. Patients at high risk of Remifentanil hydrochloride for injection abuse include those with a history of prolonged use of any opioid, including products containing remifentanil, those with a history of drug or alcohol abuse, or those who use Remifentanil hydrochloride for injection in combination with other abused drugs.
"Drug-seeking" behavior is very common in persons with substance use disorders. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing, or referral, repeated "loss" of prescriptions, tampering with prescriptions, and reluctance to provide prior medical records or contact information for other treating healthcare provider(s). "Doctor shopping" (visiting multiple prescribers to obtain additional prescriptions) is common among people who abuse drugs and people with substance use disorder. Preoccupation with achieving adequate pain relief can be appropriate behavior in a patient with inadequate pain control.
Remifentanil hydrochloride for injection, like other opioids, can be diverted for nonmedical use into illicit channels of distribution. Careful record-keeping of prescribing information, including quantity, frequency, and renewal requests, as required by state and federal law, is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic reevaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Risks Specific to Abuse of Remifentanil hydrochloride for injection
Abuse of Remifentanil hydrochloride for injection poses a risk of overdose and death. The risk is increased with concurrent use of Remifentanil hydrochloride for injection with alcohol and/or other CNS depressants.
Parenteral drug abuse is commonly associated with transmission of infectious diseases such as hepatitis and HIV.Close9.3 Dependence
Both tolerance and physical dependence can develop during use of opioid therapy.
Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose). Physical dependence is a state that develops as a result of a physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dosage reduction of a drug.
Withdrawal may be precipitated through the administration of drugs with opioid antagonist activity (e.g., naloxone), mixed agonist/antagonist analgesics (e.g., pentazocine, butorphanol, nalbuphine), or partial agonists (e.g., buprenorphine). Physical dependence may not occur to a clinically significant degree until after several days to weeks of continued use.
Remifentanil hydrochloride for injection should not be abruptly discontinued in a physically-dependent patient [see Dosage and Administration (2)]. If Remifentanil hydrochloride for injection is abruptly discontinued in a physically-dependent patient, a withdrawal syndrome may occur, typically characterized by restlessness, lacrimation, rhinorrhea, perspiration, chills, myalgia, and mydriasis. Other signs and symptoms also may develop, including irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate.
Infants born to mothers physically dependent on opioids will also be physically dependent and may exhibit respiratory difficulties and withdrawal signs [see Use in Specific Populations (8.1)]. -
10 OVERDOSAGEClinical Presentation - Acute overdose with remifentanil can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin ...
Clinical Presentation
Acute overdose with remifentanil can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, and, in some cases, pulmonary edema, bradycardia, hypotension, hypoglycemia. partial or complete airway obstruction, atypical snoring, and death. Marked mydriasis rather than miosis may be seen with hypoxia in overdose situations [see Clinical Pharmacology (12.2)].
Treatment of Overdose
In case of overdose, priorities are the reestablishment of a patent and protected airway and institution of assisted or controlled ventilation, if needed. Employ other supportive measures (including oxygen and vasopressors) in the management of circulatory shock and pulmonary edema as indicated. Cardiac arrest or arrhythmias will require advanced life-support measures.
The opioid antagonists, naloxone or nalmefene, are specific antidotes to respiratory depression resulting from opioid overdose. For clinically significant respiratory or circulatory depression secondary to remifentanil overdose, stop the infusion or administer an opioid antagonist. Opioid antagonists should not be administered in the absence of clinically significant respiratory or circulatory depression secondary to remifentanil overdose.
Close
In an individual physically dependent on opioids, administration of the recommended usual dosage of the antagonist will precipitate an acute withdrawal syndrome. The severity of the withdrawal symptoms experienced will depend on the degree of physical dependence and the dose of the antagonist administered. If a decision is made to treat serious respiratory depression in the physically dependent patient, administration of the antagonist should be begun with care and by titration with smaller than usual doses of the antagonist. -
11 DESCRIPTIONRemifentanil hydrochloride for injection is an opioid agonist. The chemical name is 3-[4-methoxycarbonyl-4-[(1-oxopropyl)phenylamino]-1-piperidine]propanoic acid methyl ester, hydrochloride salt ...
Remifentanil hydrochloride for injection is an opioid agonist. The chemical name is 3-[4-methoxycarbonyl-4-[(1-oxopropyl)phenylamino]-1-piperidine]propanoic acid methyl ester, hydrochloride salt. The molecular weight is 412.91. Its molecular formula is C20H28N2O5•HCl, and it has the following chemical structure.

Remifentanil hydrochloride for injection is a sterile, nonpyrogenic, preservative-free, white to off-white lyophilized compact powder for intravenous (IV) administration after reconstitution and dilution. Each vial contains 1 mg, 2 mg, or 5 mg of remifentanil base; 15 mg glycine; and hydrochloric acid to buffer the solutions to a nominal pH of 3 after reconstitution. When reconstituted as directed, solutions of Remifentanil hydrochloride for injection are clear, colorless solution and contain remifentanil hydrochloride (HCl) equivalent to 1 mg/mL of remifentanil base. The pH of reconstituted solutions of Remifentanil hydrochloride for injection ranges from 2.5 to 3.5. Remifentanil hydrochloride has a pKa of 6.96. Remifentanil hydrochloride has an n-octanol:water partition coefficient of 1.59.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Remifentanil hydrochloride for injection is a μ-opioid agonist with rapid onset and peak effect, and short duration of action. The μ-opioid activity of Remifentanil ...
12.1 Mechanism of Action
Remifentanil hydrochloride for injection is a μ-opioid agonist with rapid onset and peak effect, and short duration of action. The μ-opioid activity of Remifentanil hydrochloride for injection is antagonized by opioid antagonists such as naloxone.
Unlike other opioids, Remifentanil hydrochloride for injection is rapidly metabolized by hydrolysis of the propanoic acid-methyl ester linkage by nonspecific blood and tissue esterases. Remifentanil hydrochloride for injection is not a substrate for plasma cholinesterase (pseudocholinesterase) and, therefore, patients with atypical cholinesterase are expected to have a normal duration of action.
12.2 Pharmacodynamics
The analgesic effects of Remifentanil hydrochloride for injection are rapid in onset and offset. Its effects and side effects are dose dependent and similar to other μ-opioids. Remifentanil hydrochloride for injection in humans has a rapid blood-brain equilibration half-time of 1 ± 1 minutes (mean ± SD) and a rapid onset of action. The pharmacodynamic effects of Remifentanil hydrochloride for injection closely follow the measured blood concentrations, allowing direct correlation between dose, blood levels, and response. Blood concentration decreases 50% in 3 to 6 minutes after a 1-minute infusion or after prolonged continuous infusion due to rapid distribution and elimination processes and is independent of duration of drug administration. Recovery from the effects of Remifentanil hydrochloride for injection occurs rapidly (within 5 to 10 minutes). New steady-state concentrations occur within 5 to 10 minutes after changes in infusion rate. When used as a component of an anesthetic technique, Remifentanil hydrochloride for injection can be rapidly titrated to the desired depth of anesthesia/analgesia (e.g., as required by varying levels of intraoperative stress) by changing the continuous infusion rate or by administering an IV bolus injection.
Effects on the Central Nervous System
Remifentanil produces respiratory depression by direct action on brain stem respiratory centers. The respiratory depression involves both a reduction in the responsiveness of the brain stem respiratory centers to increases in carbon dioxide tension and to electrical stimulation.
Remifentanil causes miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origins may produce similar findings). Marked mydriasis rather than miosis may be seen due to hypoxia in overdose situations.
Effects on the Gastrointestinal Tract and Other Smooth Muscle
Remifentanil causes a reduction in motility associated with an increase in smooth muscle tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased to the point of spasm resulting in constipation. Other opioid-induced effects may include a reduction in biliary and pancreatic secretions, spasm of sphincter of Oddi, and transient elevations in serum amylase.
Effects on the Cardiovascular System
Remifentanil produces peripheral vasodilation which may result in orthostatic hypotension or syncope. Manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes and sweating and/or orthostatic hypotension. Caution must be used in hypovolemic patients, such as those suffering acute myocardial infarction, because remifentanil may cause or further aggravate their hypotension. Caution must also be used in patients with cor pulmonale who have received therapeutic doses of opioids.
Effects on the Endocrine System
Opioids inhibit the secretion of adrenocorticotropic hormone (ACTH), cortisol, and luteinizing hormone (LH) in humans. They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon.
Effects on the Immune System
Opioids have been shown to have a variety of effects on components of the immune system in in vitro and animal models. The clinical significance of these findings is unknown. Overall, the effects of opioids appear to be modestly immunosuppressive.
Concentration–Efficacy Relationships
The minimum effective analgesic concentration will vary widely among patients, especially among patients who have been previously treated with opioid agonists [see Dosage and Administration (2.1, 2.2)]. The minimum effective analgesic concentration of remifentanil for any individual patient may increase over time due to an increase in pain, the development of a new pain syndrome and/or the development of analgesic tolerance.
Concentration–Adverse Reaction RelationshipsThere is a relationship between increasing remifentanil plasma concentration and increasing frequency of dose-related opioid adverse reactions such as nausea, vomiting, CNS effects, and respiratory depression. In opioid-tolerant patients, the situation may be altered by the development of tolerance to opioid-related adverse reactions [see Dosage and Administration (2.1, 2.2)].
Hemodynamics
In premedicated patients undergoing anesthesia, 1-minute infusions of < 2 mcg/kg of Remifentanil hydrochloride for injection cause dose-dependent hypotension and bradycardia. While additional doses > 2 mcg/kg (up to 30 mcg/kg) do not produce any further decreases in heart rate or blood pressure, the duration of the hemodynamic change is increased in proportion to the blood concentrations achieved. Peak hemodynamic effects occur within 3 to 5 minutes of a single dose of Remifentanil hydrochloride for injection or an infusion rate increase. Glycopyrrolate, atropine, and vagolytic neuromuscular blocking agents attenuate the hemodynamic effects associated with Remifentanil hydrochloride for injection. When appropriate, bradycardia and hypotension can be reversed by reduction of the rate of infusion of Remifentanil hydrochloride for injection, or the dose of concurrent anesthetics, or by the administration of fluids or vasopressors.
Respiration
Remifentanil hydrochloride for injection depresses respiration in a dose-related fashion. Unlike other fentanyl analogs, the duration of action of Remifentanil hydrochloride for injection at a given dose does not increase with increasing duration of administration, due to lack of drug accumulation. When Remifentanil hydrochloride for injection and alfentanil were dosed to equal levels of respiratory depression, recovery of respiratory drive after 3-hour infusions was more rapid and less variable with Remifentanil hydrochloride for injection (see Figure 1).
Figure 1: Recovery of Respiratory Drive After Equipotent* Doses of Remifentanil hydrochloride for injection and Alfentanil Using CO2-Stimulated Minute Ventilation in Adult Volunteers (±1.5 SEM)

*Equipotent refers to level of respiratory depression
Spontaneous respiration occurs at blood concentrations of 4 to 5 ng/mL in the absence of other anesthetic agents; for example, after discontinuation of a 0.25 mcg/kg/min infusion of remifentanil, these blood concentrations would be reached in 2 to 4 minutes. In patients undergoing general anesthesia, the rate of respiratory recovery depends upon the concurrent anesthetic; N2O < propofol < isoflurane [see Clinical Studies: Recovery (14.2)].
Muscle Rigidity
Skeletal muscle rigidity can be caused by Remifentanil hydrochloride for injection and is related to the dose and speed of administration. Remifentanil hydrochloride for injection may cause chest wall rigidity (inability to ventilate) after single doses of > 1 mcg/kg administered over 30 to 60 seconds or infusion rates > 0.1 mcg/kg/min; peripheral muscle rigidity may occur at lower doses. Administration of doses < 1 mcg/kg may cause chest wall rigidity when given concurrently with a continuous infusion of Remifentanil hydrochloride for injection.
Histamine Release
Assays of histamine in patients and normal volunteers have shown no elevation in plasma histamine levels after administration of Remifentanil hydrochloride for injection in doses up to 30 mcg/kg over 60 seconds.
Analgesia
Infusions of 0.05 to 0.1 mcg/kg/min, producing blood concentrations of 1 to 3 ng/mL, are typically associated with analgesia with minimal decrease in respiratory rate. Supplemental doses of 0.5 to 1 mcg/kg, incremental increases in infusion rate > 0.05 mcg/kg/min, and blood concentrations exceeding 5 ng/mL (typically produced by infusions of 0.2 mcg/kg/min) have been associated with transient and reversible respiratory depression, apnea, and muscle rigidity.
Anesthesia
Remifentanil hydrochloride for injection is synergistic with the activity of hypnotics (propofol and thiopental), inhaled anesthetics, and benzodiazepines [see Clinical Studies (14.1), Warnings and Precautions (5), and Dosage and Administration (2)].
Age
The pharmacodynamic activity of Remifentanil hydrochloride for injection (as measured by the EC50 for development of delta waves on the EEG) increases with increasing age. The EC50 of remifentanil for this measure was 50% less in patients over 65 years of age when compared to healthy volunteers (25 years of age) [see Dosage and Administration (2.2)].
Sex
No differences have been shown in the pharmacodynamic activity (as measured by the EEG) of Remifentanil hydrochloride for injection between men and women.
Drug Interactions
In animals the duration of muscle paralysis from succinylcholine is not prolonged by remifentanil.
Intraocular Pressure
There was no change in intraocular pressure after the administration of Remifentanil hydrochloride for injection prior to ophthalmic surgery under monitored anesthesia care.
Cerebrodynamics
Under isoflurane-nitrous oxide anesthesia (PaCO2 < 30 mmHg), a 1-minute infusion of Remifentanil hydrochloride for injection (0.5 or 1.0 mcg/kg) produced no change in intracranial pressure. Mean arterial pressure and cerebral perfusion decreased as expected with opioids. In patients receiving Remifentanil hydrochloride for injection and nitrous oxide anesthesia, cerebrovascular reactivity to carbon dioxide remained intact. In humans, no epileptiform activity was seen on the EEG (n = 44) at remifentanil doses up to 8 mcg/kg/min.
Renal Dysfunction
The pharmacodynamics of Remifentanil hydrochloride for injection (ventilatory response to hypercarbia) are unaltered in patients with end stage renal disease (creatinine clearance < 10 mL/min).
Hepatic Dysfunction
The pharmacodynamics of Remifentanil hydrochloride for injection (ventilatory response to hypercarbia) are unaltered in patients with severe hepatic dysfunction awaiting liver transplant.
Close12.3 Pharmacokinetics
After IV doses administered over 60 seconds, the pharmacokinetics of remifentanil fit a three-compartment model with a rapid distribution half-life of one minute, a slower distribution half-life of 6 minutes, and a terminal elimination half-life of 10 to 20 minutes. Since the terminal elimination component contributes less than 10% of the overall area under the concentration versus time curve (AUC), the effective biological half-life of Remifentanil hydrochloride for injection is 3 to 10 minutes. This is similar to the 3- to 10-minute half-life measured after termination of prolonged infusions (up to 4 hours; see Figure 2) and correlates with recovery times observed in the clinical setting after infusions up to 12 hours. Concentrations of remifentanil are proportional to the dose administered throughout the recommended dose range. The pharmacokinetics of remifentanil are unaffected by the presence of renal or hepatic impairment.
Distribution
The initial volume of distribution (Vd) of remifentanil is approximately 100 mL/kg and represents distribution throughout the blood and rapidly perfused tissues. Remifentanil subsequently distributes into peripheral tissues with a steady-state volume of distribution of approximately 350 mL/kg. These two distribution volumes generally correlate with total body weight (except in severely obese patients when they correlate better with ideal body weight [IBW]). Remifentanil is approximately 70% bound to plasma proteins of which two-thirds is binding to alpha-1-acid-glycoprotein.
Elimination
The clearance of remifentanil in young, healthy adults is approximately 40 mL/min/kg. Clearance generally correlates with total body weight (except in severely obese patients when it correlates better with IBW). The high clearance of remifentanil combined with a relatively small volume of distribution produces a short elimination half-life of approximately 3 to 10 minutes (see Figure 2). This value is consistent with the time taken for blood or effect site concentrations to fall by 50% (context-sensitive half-times) which is approximately 3 to 6 minutes. Unlike other fentanyl analogs, the duration of action does not increase with prolonged administration.
Figure 2: Mean Concentration (sd) versus Time

Titration to Effect
The rapid elimination of remifentanil permits the titration of infusion rate without concern for prolonged duration. In general, every 0.1 mcg/kg/min change in the IV infusion rate will lead to a corresponding 2.5 ng/mL change in blood remifentanil concentration within 5 to 10 minutes. In intubated patients only, a more rapid increase (within 3 to 5 minutes) to a new steady state can be achieved with a 1.0 mcg/kg bolus dose in conjunction with an infusion rate increase.
Metabolism
Remifentanil is an esterase-metabolized opioid. A labile ester linkage renders this compound susceptible to hydrolysis by nonspecific esterases in blood and tissues. This hydrolysis results in the production of the carboxylic acid metabolite (3-[4-methoxycarbonyl-4-[(1-oxopropyl)phenylamino]-1-piperidine]propanoic acid), and represents the principal metabolic pathway for remifentanil (> 95%). The carboxylic acid metabolite is essentially inactive (1/4600 as potent as remifentanil in dogs). Remifentanil is not metabolized by plasma cholinesterase (pseudocholinesterase) and is not appreciably metabolized by the liver or lung.
Excretion
The carboxylic acid metabolite is excreted by the kidneys with an elimination half-life of approximately 90 minutes.
Specific Populations
Age: Geriatric Population
The clearance of remifentanil is reduced (approximately 25%) in the elderly (> 65 years of age) compared to young adults (average 25 years of age). However, remifentanil blood concentrations fall as rapidly after termination of administration in the elderly as in young adults.
Age: Pediatric Population
In pediatric patients, 5 days to 17 years of age (n = 47), the clearance and volume of distribution of remifentanil were increased in younger children and declined to young healthy adult values by age 17. The average clearance of remifentanil in neonates (less than 2 months of age) was approximately 90.5 ± 36.8 mL/min/kg (mean ± SD) while in adolescents (13 to 16 years) this value was 57.2 ± 21.1 mL/min/kg. The total (steady-state) volume of distribution in neonates was 452 ± 144 mL/kg versus 223 ± 30.6 mL/kg in adolescents. The half-life of remifentanil was the same in neonates and adolescents. Clearance of remifentanil was maintained at or above normal adult values in patients 5 days to 17 years of age.
Sex
There is no significant difference in the pharmacokinetics of remifentanil in male and female patients after correcting for differences in weight.
Hepatic Impairment
The pharmacokinetics of remifentanil and its carboxylic acid metabolite are unchanged in patients with severe hepatic impairment.
Renal Impairment
The pharmacokinetic profile of Remifentanil hydrochloride for injection is not changed in patients with end stage renal disease (creatinine clearance < 10 mL/min). In anephric patients, the half-life of the carboxylic acid metabolite increases from 90 minutes to 30 hours. The metabolite is removed by hemodialysis with a dialysis extraction ratio of approximately 30%.
Obesity
There is no difference in the pharmacokinetics of remifentanil in non-obese versus obese (greater than 30% over IBW) patients when normalized to IBW.
Cardiopulmonary Bypass (CPB)
Remifentanil clearance is reduced by approximately 20% during hypothermic CPB.
Drug Interaction Studies
Remifentanil clearance is not altered by concomitant administration of thiopental, isoflurane, propofol, or temazepam during anesthesia. In vitro studies with atracurium, mivacurium, esmolol, echothiophate, neostigmine, physostigmine, and midazolam revealed no inhibition of remifentanil hydrolysis in whole human blood by these drugs.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term studies in animals to evaluate the carcinogenic potential of remifentanil have not been conducted ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals to evaluate the carcinogenic potential of remifentanil have not been conducted.
Mutagenesis
Mutagenicity was observed with remifentanil in the in vitro mouse lymphoma assay in the presence but not absence of metabolic activation. Remifentanil did not induce gene mutation in the in vitro bacterial reverse mutation assay (Ames test) and was not genotoxic in the in vivo rat hepatocyte unscheduled DNA synthesis assay. No clastogenic effect was seen in cultured Chinese hamster ovary cells or in the in vivo mouse micronucleus test.
Impairment of Fertility
Remifentanil has been shown to reduce fertility in male rats when tested after 70+ days of daily IV administration of 0.5 mg/kg, which is approximately 0.2 times a human intravenous infusion of an induction dose of 1 mcg/kg with a maintenance dose of 2 mcg/kg/min in terms of mg/m2 of body surface area for a surgical procedure lasting 3 hours or 40 times a single bolus human dose of 2 mcg/kg, in terms of mg/m2 of body surface area.
The fertility of female rats was not affected at IV doses as high as 1 mg/kg which is 0.4 times a human intravenous infusion of an induction dose of 1 mcg/kg with a maintenance dose of 2 mcg/kg/min in terms of mg/m2 of body surface area for a surgical procedure lasting 3 hours or approximately 80 times a single bolus human dose of 2 mcg/kg, in terms of mg/m2 of body surface area, when administered for at least 15 days before mating.
-
14 CLINICAL STUDIESRemifentanil hydrochloride for injection was evaluated in 3,341 patients undergoing general anesthesia (n = 2,706) and monitored anesthesia care (n = 639). These patients were evaluated in the ...
Remifentanil hydrochloride for injection was evaluated in 3,341 patients undergoing general anesthesia (n = 2,706) and monitored anesthesia care (n = 639). These patients were evaluated in the following settings: inpatient (n = 2,079) which included cardiovascular (n = 426), and neurosurgical (n = 61), and outpatient (n = 1,349). Four-hundred and eighty-six (486) elderly patients (age range 66 to 90 years) and 410 pediatric patients (age range birth to 12 years) received Remifentanil hydrochloride for injection. Of the general anesthesia patients, 682 also received Remifentanil hydrochloride for injection as an IV analgesic agent during the immediate postoperative period.
14.1 Induction and Maintenance of General Anesthesia - Inpatient/Outpatient
The efficacy of Remifentanil hydrochloride for injection was investigated in 1,562 patients in 15 randomized, controlled trials as the analgesic component for the induction and maintenance of general anesthesia. Eight of these studies compared Remifentanil hydrochloride for injection to alfentanil and two studies compared Remifentanil hydrochloride for injection to fentanyl. In these studies, doses of Remifentanil hydrochloride for injection up to the ED90 were compared to recommended doses (approximately ED50) of alfentanil or fentanyl.
Induction of Anesthesia
Remifentanil hydrochloride for injection was administered with isoflurane, propofol, or thiopental for the induction of anesthesia (n = 1,562). The majority of patients (80%) received propofol as the concurrent agent. Remifentanil hydrochloride for injection reduced the propofol and thiopental requirements for loss of consciousness. Compared to alfentanil and fentanyl, a higher relative dose of Remifentanil hydrochloride for injection resulted in fewer responses to intubation (see Table 19). Overall, hypotension occurred in 5% of patients receiving Remifentanil hydrochloride for injection compared to 2% of patients receiving the other opioids.
Remifentanil hydrochloride for injection has been used as a primary agent for the induction of anesthesia; however, it should not be used as a sole agent because loss of consciousness cannot be assured and because of a high incidence of apnea, muscle rigidity, and tachycardia. The administration of an induction dose of propofol or thiopental or a paralyzing dose of a muscle relaxant prior to or concurrently with Remifentanil hydrochloride for injection during the induction of anesthesia markedly decreased the incidence of muscle rigidity from 20% to < 1%.
Table 19: Response to Intubation (Propofol/Opioid Inductiona)
Opioid Treatment Group/ (No. of Patients)
Initial Dose (mcg/kg)
Pre-Intubation Infusion Rate (mcg/kg/min)
No. (%) Muscle Rigidity
No. (%) Hypotension During Induction
No. (%) Response to Intubation
Study 1:
Remifentanil hydrochloride for injection (35)
1
0.1
1 (3%)
0
27 (77%)
Remifentanil hydrochloride for injection (35)
1
0.4
3 (9%)
0
11 (31%)b
Alfentanil (35)
20
1.0
2 (6%)
0
26 (74%)
Study 2:
Remifentanil hydrochloride for injection (116)
1
0.5
9 (8%)
5 (4%)
17 (15%)b
Alfentanil (118)
25
1.0
6 (5%)
5 (4%)
33 (28%)
Study 3:
Remifentanil hydrochloride for injection (134)
1
0.5
2 (1%)
4 (3%)
25 (19%)
Alfentanil (66)
20
2.0
0
0
19 (29%)
Study 4:
Remifentanil hydrochloride for injection (98)
1
0.2
11 (11%)b
2 (2%)
35 (36%)
Remifentanil hydrochloride for injection (91)
2c
0.4
11 (12%)b
2 (2%)
12 (13%)b
Fentanyl (97)
3
NA
1 (1%)
1 (1%)
29 (30%)
a Propofol was titrated to loss of consciousness. Not all doses of Remifentanil hydrochloride for injection were equipotent to the comparator opioid.
b Differences were statistically significant (P < 0.02).
c Initial doses greater than 1 mcg/kg are not recommended.
Use During Maintenance of Anesthesia
Remifentanil hydrochloride for injection was investigated in 929 patients in seven well controlled general surgery studies in conjunction with nitrous oxide, isoflurane, or propofol in both inpatient and outpatient settings. These studies demonstrated that Remifentanil hydrochloride for injection could be dosed to high levels of opioid effect and rapidly titrated to optimize analgesia intraoperatively without delaying or prolonging recovery.
Compared to alfentanil and fentanyl, these higher relative doses (ED90) of Remifentanil hydrochloride for injection resulted in fewer responses to intraoperative stimuli (see Table 20) and a higher frequency of hypotension (16% compared to 5% for the other opioids). Remifentanil hydrochloride for injection was infused to the end of surgery, while alfentanil was discontinued 5 to 30 minutes before the end of surgery as recommended. The mean final infusion rates of Remifentanil hydrochloride for injection were between 0.25 and 0.48 mcg/kg/min.
Table 20: Intraoperative Responsesa
Opioid Treatment Group/(No. of Patients)
Concurrent Anesthetic
Post-Intubation Infusion Rate (mcg/kg/min)
No. (%) With Intraoperative Hypotension
No. (%) With Response to Skin Incision
No. (%) With Signs of Light Anesthesia
No. (%) With Response to Skin Closure
Study 1:
Remifentanil hydrochloride for injection (35)
0.1
0
20 (57%)
33 (94%)
6 (17%)
Remifentanil hydrochloride for injection (35)
Nitrous oxide
0.4
0
3 (9%)b
12 (34%)b
2 (6%)b
Alfentanil (35)
1.0
0
24 (69%)
33 (94%)
12 (34%)
Study 2:
Remifentanil hydrochloride for injection (116)
Isoflurane +
0.25
35 (30%)b
9 (8%)b
66 (57%)b
19 (16%)
Alfentanil (118)
Nitrous oxide
0.5
12 (10%)
20 (17%)
85 (72%)
25 (21%)
Study 3:
Remifentanil hydrochloride for injection (134)
Propofol
0.5
3 (2%)
14 (11%)b
70 (52%)b
25 (19%)
Alfentanil (66)
2.0
2 (3%)
21 (32%)
47 (71%)
13 (20%)
Study 4:
Remifentanil hydrochloride for injection (98)
0.2
13 (13%)
12 (12%)b
67 (68%)b
7 (7%)
Remifentanil hydrochloride for injection (91)
Isoflurane
0.4
16 (18%)b
4 (4%)b
44 (48%)b
3 (3%)b
Fentanyl (97)
1.5-3 mcg/kg prn
7 (7%)
32 (33%)
84 (87%)
11 (11%)
a Not all doses of Remifentanil hydrochloride for injection were equipotent to the comparator opioid.
b Differences were statistically significant (P < 0.05).
In three randomized, controlled studies (n = 407) during general anesthesia, Remifentanil hydrochloride for injection attenuated the signs of light anesthesia within a median time of 3 to 6 minutes after bolus doses of 1 mcg/kg with or without infusion rate increases of 50% to 100% (up to a maximum rate of 2 mcg/kg/min).
In an additional double-blind, randomized study (n = 103), a constant rate (0.25 mcg/kg/min) of Remifentanil hydrochloride for injection was compared to doubling the rate to 0.5 mcg/kg/min approximately 5 minutes before the start of the major surgical stress event. Doubling the rate decreased the incidence of signs of light anesthesia from 67% to 8% in patients undergoing abdominal hysterectomy, and from 19% to 10% in patients undergoing radical prostatectomy. In patients undergoing laminectomy the lower dose was adequate.
14.2 Recovery
In 2,169 patients receiving Remifentanil hydrochloride for injection for periods up to 16 hours, recovery from anesthesia was rapid, predictable, and independent of the duration of the infusion of Remifentanil hydrochloride for injection. In the seven controlled, general surgery studies, extubation occurred in a median of 5 minutes (range: -3 to 17 minutes in 95% of patients) in outpatient anesthesia and 10 minutes (range: 0 to 32 minutes in 95% of patients) in inpatient anesthesia. Recovery in studies using nitrous oxide or propofol was faster than in those using isoflurane as the concurrent anesthetic. There was no case of remifentanil-induced delayed respiratory depression occurring more than 30 minutes after discontinuation of remifentanil [see Warnings and Precautions (5.15)].
In a double-blind, randomized study, administration of morphine sulfate (0.15 mg/kg) intravenously 20 minutes before the anticipated end of surgery to 98 patients did not delay recovery of respiratory drive in patients undergoing major surgery with remifentanil-propofol total IV anesthesia.
14.3 Spontaneous Ventilation Anesthesia
Two randomized, dose-ranging studies (n = 127) examined the administration of Remifentanil hydrochloride for injection to outpatients undergoing general anesthesia with a laryngeal mask. Starting infusion rates of Remifentanil hydrochloride for injection of ≤ 0.05 mcg/kg/min provided supplemental analgesia while allowing spontaneous ventilation with propofol or isoflurane. Bolus doses of Remifentanil hydrochloride for injection during spontaneous ventilation lead to transient periods of apnea, respiratory depression, and muscle rigidity.
14.4 Pediatric Anesthesia
Remifentanil hydrochloride for injection has been evaluated for maintenance of general anesthesia in 410 pediatric patients from birth to 12 years undergoing inpatient and outpatient procedures. Four clinical studies have been performed.
Study 1, an open-label, randomized, controlled clinical trial (n = 129), compared Remifentanil hydrochloride for injection (n = 68) with alfentanil (n = 19), isoflurane (n = 22), or propofol (n = 20) in children 2 to 12 years of age undergoing strabismus surgery. After induction of anesthesia which included the administration of atropine, Remifentanil hydrochloride for injection was administered as an initial infusion of 1 mcg/kg/min with 70% nitrous oxide. The infusion rate required during maintenance of anesthesia was 0.73 to 1.95 mcg/kg/min. Time to extubation and to purposeful movement was a median of 10 minutes (range 1 to 24 minutes).
Study 2, a double-blind, randomized, controlled trial (n = 222), compared Remifentanil hydrochloride for injection (n = 119) to fentanyl (n = 103) in children 2 to 12 years of age undergoing tonsillectomy with or without adenoidectomy. After induction of anesthesia, patients received a 0.25 mcg/kg/min infusion of Remifentanil hydrochloride for injection or fentanyl by IV bolus with nitrous oxide/oxygen (2:1) and either halothane or sevoflurane for maintenance of anesthesia. The mean infusion rate required during maintenance of anesthesia was 0.3 mcg/kg/min (range 0.2 to 1.3 mcg/kg/min). The continuous infusion rate was decreased to 0.05 mcg/kg/min approximately 10 minutes prior to the end of surgery. Time to spontaneous purposeful movement was a median of 8 minutes (range 1 to 19 minutes). Time to extubation was a median of 9 minutes (range 2 to 19 minutes).
Study 3, an open-label, randomized, controlled trial (n = 271), compared Remifentanil hydrochloride for injection (n = 185) with a regional anesthetic technique (n = 86) in children 1 to 12 years of age undergoing major abdominal, urological, or orthopedic surgery. Patients received a 0.25 mcg/kg/min infusion of Remifentanil hydrochloride for injection following a 1.0 mcg/kg bolus or bupivacaine by epidural infusion, along with isoflurane and nitrous oxide after the induction of anesthesia. The mean infusion rate required during maintenance of anesthesia was 0.25 mcg/kg/min (range 0 to 0.75 mcg/kg/min). Both treatments were effective in attenuating responses to skin incision during surgery. The hemodynamic profile of the Remifentanil hydrochloride for injection group was consistent with an opioid-based general anesthetic technique. Time to spontaneous purposeful movement was a median of 15 minutes (range, 2 to 75 minutes) in the remifentanil group. Time to extubation was a median of 13 minutes (range, 4 to 31 minutes) in the remifentanil group.
Study 4, an open-label, randomized, controlled trial (n = 60), compared Remifentanil hydrochloride for injection (n = 38) with halothane (n = 22) in ASA 1 or 2, full term neonates and infants ≤ 8 weeks of age weighing at least 2500 grams who were undergoing pyloromyotomy. After induction of anesthesia, which included the administration of atropine, patients received 0.4 mcg/kg/min of Remifentanil hydrochloride for injection or 0.4% halothane with 70% nitrous oxide for initial maintenance of anesthesia and then both agents were adjusted according to clinical response. Bolus doses of 1 mcg/kg administered over 30 to 60 seconds were used to treat brief episodes of hypertension and tachycardia, and infusion rates were increased by 50% to treat sustained hypertension and tachycardia. The range of infusion rates of Remifentanil hydrochloride for injection required during maintenance of anesthesia was 0.4 to 1 mcg/kg/min.
Seventy-one percent (71%) of Remifentanil hydrochloride for injection patients required supplementary boluses or rate increases from the starting dose of 0.4 mcg/kg/min to treat hypertension, tachycardia, movement or somatic signs of light anesthesia. Twenty-four percent of the patients required an increase from the initial rate of 0.4 mcg/kg/min prior to incision and 26% of patients required an infusion rate between 0.8 and 1.0 mcg/kg/min, most often during gastric manipulation. The continuous infusion rate was decreased to 0.05 mcg/kg/min approximately 10 minutes before the end of surgery.
In the Remifentanil hydrochloride for injection group, median time from discontinuation of anesthesia to spontaneous purposeful movement was 6.5 minutes (range, 1 to 13 minutes) and median time to extubation was 8.5 minutes (range, 1 to 14 minutes).
The initial maintenance infusion regimen of Remifentanil hydrochloride for injection evaluated in pediatric patients from birth to 2 months of age was 0.4 mcg/kg/min, the approved adult regimen for use with N2O. The clearance rate observed in the neonatal population was highly variable and on average was two times higher than in the young healthy adult population. [See Clinical Pharmacology: Specific Populations: Pediatric Population (12.3) and Dosage and Administration, Table 2 (2.2).]
No pediatric patients receiving Remifentanil hydrochloride for injection required naloxone during the immediate postoperative recovery period.
14.5 Coronary Artery Bypass Surgery
Remifentanil hydrochloride for injection was originally administered to 225 subjects undergoing elective CABG surgery in two dose-ranging studies without active comparators. Subsequently, two double-blind, double-dummy clinical studies (N = 426) evaluated Remifentanil hydrochloride for injection (n = 236) at recommended doses versus active comparators (n = 190).
The first comparator study, a multi-center, randomized, double-blind, double-dummy, parallel-group study (N = 369), compared Remifentanil hydrochloride for injection (n = 201) with fentanyl (n = 168) in adult patients undergoing elective CABG surgery. Subjects received 1 to 3 mg midazolam and 0.05 mg/kg morphine IV as premedication. Anesthesia was induced with propofol 0.5 mg/kg (higher doses administered with Remifentanil hydrochloride for injection were associated with excessive hypotension) over one minute plus 10-mg boluses every 10 seconds until loss of consciousness followed by either cisatracurium 0.2 mg/kg or vecuronium 0.15 mg/kg. Patients randomized to Remifentanil hydrochloride for injection received a 1 mcg/kg/min infusion of Remifentanil hydrochloride for injection followed by a placebo bolus administered over 3 minutes. In the active control group, a placebo IV infusion was started and a fentanyl bolus 10 mcg/kg was administered over 3 minutes. All subjects received isoflurane titrated initially to end tidal concentration of 0.5%. During maintenance, the group randomized to Remifentanil hydrochloride for injection received as needed 0.5-1 mcg/kg/min IV rate increases (to a maximum of 4 mcg/kg/min) of Remifentanil hydrochloride for injection and 1 mcg/kg IV boluses of Remifentanil hydrochloride for injection. The active control group received 2 mcg/kg IV boluses of fentanyl and increases in placebo IV infusion rate.
The second comparator study, a multi-center, double-blind, randomized, parallel group study (N = 57), compared Remifentanil hydrochloride for injection (n = 35) to fentanyl (n = 22) in adult patients undergoing elective CABG surgery with poor left ventricular function (ejection fraction < 0.35). Subjects received oral lorazepam 40 mcg/kg as premedication. Anesthesia was induced using etomidate until loss of consciousness, followed by a low-dose propofol infusion (3 mg/kg/hr) and pancuronium 0.15 mg/kg. Subjects in the group administered Remifentanil hydrochloride for injection received a placebo bolus dose and a continuous infusion of Remifentanil hydrochloride for injection 1 mcg/kg/min and subjects in the fentanyl group received a bolus loading dose of 15 mcg/kg and placebo continuous infusion. During maintenance, supplemental bolus doses of Remifentanil hydrochloride for injection (0.5 mcg/kg) and infusion rate increases of 0.5 to 1 mcg/kg/min (maximum rate allowed was 4 mcg/kg/min) of Remifentanil hydrochloride for injection were administered to one group; while the fentanyl group was given intermittent maintenance bolus doses of 2 mcg/kg and increases in the placebo infusion rate.
In these two studies, using a high dose opioid technique with Remifentanil hydrochloride for injection as a component of a balanced or total intravenous anesthetic regimen, the remifentanil regimen effectively attenuated response to maximal sternal spread generally better than the dose and regimen studied for the active control (fentanyl). While this provides evidence for the efficacy of remifentanil as an analgesic in this setting, caution must be exercised in interpreting these results as evidence of superiority of remifentanil over the active control, since these studies did not make any attempt to evaluate and compare the optimal analgesic doses of either drug in this setting.
14.6 Neurosurgery
Remifentanil hydrochloride for injection was administered to 61 patients undergoing craniotomy for removal of a supratentorial mass lesion. In these studies, ventilation was controlled to maintain a predicted PaCO2 of approximately 28 mmHg. In one study (n = 30) with Remifentanil hydrochloride for injection and 66% nitrous oxide, the median time to extubation and to patient response to verbal commands was 5 minutes (range -1 to 19 minutes). Intracranial pressure and cerebrovascular responsiveness to carbon dioxide were normal [see Clinical Pharmacology (12.2)].
A randomized, controlled study compared Remifentanil hydrochloride for injection (n = 31) to fentanyl (n = 32). Remifentanil hydrochloride for injection (1 mcg/kg/min) and fentanyl (2 mcg/kg/min) were administered after induction with thiopental and pancuronium. A similar number of patients (6%) receiving Remifentanil hydrochloride for injection and fentanyl had hypotension during induction. Anesthesia was maintained with nitrous oxide and Remifentanil hydrochloride for injection at a mean infusion rate of 0.23 mcg/kg/min (range 0.1 to 0.4) compared with a fentanyl mean infusion rate of 0.04 mcg/kg/min (range 0.02 to 0.07). Supplemental isoflurane was administered as needed. The patients receiving Remifentanil hydrochloride for injection required a lower mean isoflurane dose (0.07 MAC-hours) compared with 0.64 MAC-hours for the fentanyl patients (P = 0.04). Remifentanil hydrochloride for injection was discontinued at the end of anesthesia, whereas fentanyl was discontinued at the time of bone flap replacement (a median time of 44 minutes before the end of surgery). Median time to extubation was similar (5 and 3.5 minutes, respectively, with Remifentanil hydrochloride for injection and fentanyl). None of the patients receiving Remifentanil hydrochloride for injection required naloxone compared to seven of the fentanyl patients (P = 0.01). Eighty-one percent (81%) of patients receiving Remifentanil hydrochloride for injection recovered (awake, alert, and oriented) within 30 minutes after surgery compared with 59% of fentanyl patients (P = 0.06). At 45 minutes, recovery rates were similar (81% and 69% respectively for Remifentanil hydrochloride for injection and fentanyl, P = 0.27). Patients receiving Remifentanil hydrochloride for injection required an analgesic for headache sooner than fentanyl patients (median of 35 minutes compared with 136 minutes, respectively [P = 0.04]). No adverse cerebrovascular effects were seen in this study [see Clinical Pharmacology (12.2)].
14.7 Continuation of Analgesic Use into the Immediate Postoperative Period
Analgesia with Remifentanil hydrochloride for injection in the immediate postoperative period (until approximately 30 minutes after extubation) was studied in 401 patients in four dose-finding studies and in 281 patients in two efficacy studies. In the dose-finding studies, the use of bolus doses of Remifentanil hydrochloride for injection and incremental infusion rate increases ≥ 0.05 mcg/kg/min led to respiratory depression and muscle rigidity.
In two efficacy studies, Remifentanil hydrochloride for injection 0.1 mcg/kg/min was started immediately after discontinuing anesthesia. Incremental infusion rate increases of 0.025 mcg/kg/min every 5 minutes were given to treat moderate to severe postoperative pain. In Study 1, 50% decreases in infusion rate were made if respiratory rate decreased below 12 breaths/min and in Study 2, the same decreases were made if respiratory rate was below 8 breaths/min. With this difference in criteria for infusion rate decrease, the incidence of respiratory depression was lower in Study 1 (4%) than in Study 2 (12%). In both studies, Remifentanil hydrochloride for injection provided effective analgesia (no or mild pain with respiratory rate ≥ 8 breaths/min) in approximately 60% of patients at mean final infusion rates of 0.1 to 0.125 mcg/kg/min.
Study 2 was a double-blind, randomized, controlled study in which patients received either morphine sulfate (0.15 mg/kg administered 20 minutes before the anticipated end of surgery plus 2 mg bolus doses for supplemental analgesia) or Remifentanil hydrochloride for injection (as described above). Emergence from anesthesia was similar between groups; median time to extubation was 5 to 6 minutes for both. Remifentanil hydrochloride for injection provided effective analgesia in 58% of patients compared to 33% of patients who received morphine. Respiratory depression occurred in 12% of patients receiving Remifentanil hydrochloride for injection compared to 4% of morphine patients. For patients who received Remifentanil hydrochloride for injection, morphine sulfate (0.15 mg/kg) was administered in divided doses 5 and 10 minutes before discontinuing Remifentanil hydrochloride for injection. Within 30 minutes after discontinuation of Remifentanil hydrochloride for injection, the percentage of patients with effective analgesia decreased to 34%.
Close14.8 Monitored Anesthesia Care
Remifentanil hydrochloride for injection has been studied in the monitored anesthesia care setting in 609 patients in eight clinical studies. Nearly all patients received supplemental oxygen in these studies. Two early dose-finding studies demonstrated that use of sedation as an endpoint for titration of Remifentanil hydrochloride for injection led to a high incidence of muscle rigidity (69%) and respiratory depression. Subsequent trials titrated Remifentanil hydrochloride for injection to specific clinical endpoints of patient comfort, analgesia, and adequate respiration (respiratory rate > 8 breaths/min) with a corresponding lower incidence of muscle rigidity (3%) and respiratory depression. With doses of midazolam > 2 mg (4 to 8 mg), the dose of Remifentanil hydrochloride for injection could be decreased by 50%, but the incidence of respiratory depression rose to 32%.
The efficacy of a single dose of Remifentanil hydrochloride for injection (1.0 mcg/kg over 30 seconds) was compared to alfentanil (7 mcg/kg over 30 seconds) in patients undergoing ophthalmic surgery. More patients receiving Remifentanil hydrochloride for injection were pain free at the time of the nerve block (77% versus 44%, P = 0.02) and more experienced nausea (12% versus 4%) than those receiving alfentanil.
In a randomized, controlled study (n = 118), Remifentanil hydrochloride for injection 0.5 mcg/kg over 30 to 60 seconds followed by a continuous infusion of 0.1 mcg/kg/min, was compared to a propofol bolus (500 mcg/kg) followed by a continuous infusion (50 mcg/kg/min) in patients who received a local or regional anesthetic nerve block 5 minutes later. The incidence of moderate or severe pain during placement of the block was similar between groups (2% with Remifentanil hydrochloride for injection and 8% with propofol, P = 0.2) and more patients receiving Remifentanil hydrochloride for injection experienced nausea (26% versus 2%, P < 0.001). The final mean infusion rate of Remifentanil hydrochloride for injection was 0.08 mcg/kg/min.
In a randomized, double-blind study, Remifentanil hydrochloride for injection with or without midazolam was evaluated in 159 patients undergoing superficial surgical procedures under local anesthesia. Remifentanil hydrochloride for injection was administered without midazolam as a 1 mcg/kg dose over 30 seconds followed by a continuous infusion of 0.1 mcg/kg/min. In the group of patients that received midazolam, Remifentanil hydrochloride for injection was administered as a 0.5 mcg/kg dose over 30 seconds followed by a continuous infusion of 0.05 mcg/kg/min and midazolam 2 mg was administered 5 minutes later. The occurrence of moderate or severe pain during the local anesthetic injection was similar between groups (16% and 20%). Other effects for Remifentanil hydrochloride for injection alone and Remifentanil hydrochloride for injection /midazolam were: respiratory depression with oxygen desaturation (SPO2 < 90%), 5% and 2%; nausea, 8% and 2%; and pruritus, 23% and 12%. Titration of Remifentanil hydrochloride for injection resulted in prompt resolution of respiratory depression (median 3 minutes, range 0 to 6 minutes). The final mean infusion rate of Remifentanil hydrochloride for injection was 0.12 mcg/kg/min (range 0.03 to 0.3) for the group receiving Remifentanil hydrochloride for injection alone and 0.07 mcg/kg/min (range 0.02 to 0.2) for the group receiving Remifentanil hydrochloride for injection /midazolam.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGRemifentanil hydrochloride for injection, for intravenous use, is supplied as follows: NDC Number - Container - Concentration - Quantity - 75834-230-10 - 3 mL Single-Dose ...
Remifentanil hydrochloride for injection, for intravenous use, is supplied as follows:
NDC Number
Container
Concentration
Quantity
75834-230-10
3 mL Single-Dose Vial
1 mg lyophilized powder
Packaged in cartons of 10
75834-231-10
5 mL Single-Dose Vial
2 mg lyophilized powder
Packaged in cartons of 10
75834-232-10
10 mL Single-Dose Vial
5 mg lyophilized powder
Packaged in cartons of 10
Remifentanil hydrochloride for injection should be stored at 2° to 25°C (36° to 77°F).
Discard unused portion.
Close -
17 PATIENT COUNSELING INFORMATIONAddiction, Abuse, and Misuse - Inform patients that the use of Remifentanil hydrochloride for injection, even when taken as recommended, can result in addiction, abuse, and misuse, which can lead ...
Addiction, Abuse, and Misuse
Inform patients that the use of Remifentanil hydrochloride for injection, even when taken as recommended, can result in addiction, abuse, and misuse, which can lead to overdose and death [see Warnings and Precautions (5.1)].
Life-Threatening Respiratory Depression
Inform patients of the risk of life-threatening respiratory depression, including information that the risk is greatest when starting Remifentanil hydrochloride for injection or when the dosage is increased, and that it can occur even at recommended dosages.
Hyperalgesia and Allodynia
Advise patients to inform their healthcare provider if they experience symptoms of hyperalgesia, including worsening pain, increased sensitivity to pain, or new pain [see Warnings and Precautions (5.4), Adverse Reactions (6)].
Serotonin Syndrome
Inform patients that opioids could cause a rare but potentially life-threatening condition called serotonin syndrome resulting from concomitant administration of serotonergic drugs. Warn patients of the symptoms of serotonin syndrome and to seek medical attention right away if symptoms develop after discharge from the hospital. Instruct patients to inform their healthcare provider if they are taking, or plan to take serotonergic medications [see Warnings and Precautions (5.5), Drug Interactions (7)].
Constipation
Advise patients of the potential for severe constipation, including management instructions and when to seek medical attention [see Adverse Reactions (6), Clinical Pharmacology (12.2)].
Manufactured for:
Nivagen Pharmaceuticals, Inc.
Sacramento, CA 95827, USA
Toll free number: 1-877-977-0687
Revised: 4/2024
Close -
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC code 75834-230-01 - Remifentanil Hydrochloride for Injection 1 mg - Vial Label 1 mg - NDC code 75834-231-01 - Remifentanil Hydrochloride for Injection 2 mg - Vial Label 2 mg ...
NDC code 75834-230-01
Remifentanil Hydrochloride for Injection 1 mg
Vial Label 1 mg

NDC code 75834-231-01
Remifentanil Hydrochloride for Injection 2 mg
Vial Label 2 mg

NDC code 75834-232-01
Remifentanil Hydrochloride for Injection 5 mg
Vial Label 5 mg

NDC code 75834-230-10
Remifentanil Hydrochloride for Injection 1 mg
Vial Carton 1 mg

NDC code 75834-231-10
Remifentanil Hydrochloride for Injection 2 mg
Vial Carton 2 mg

NDC code 75834-232-10
Remifentanil Hydrochloride for Injection 5 mg
Vial Carton 5 mg
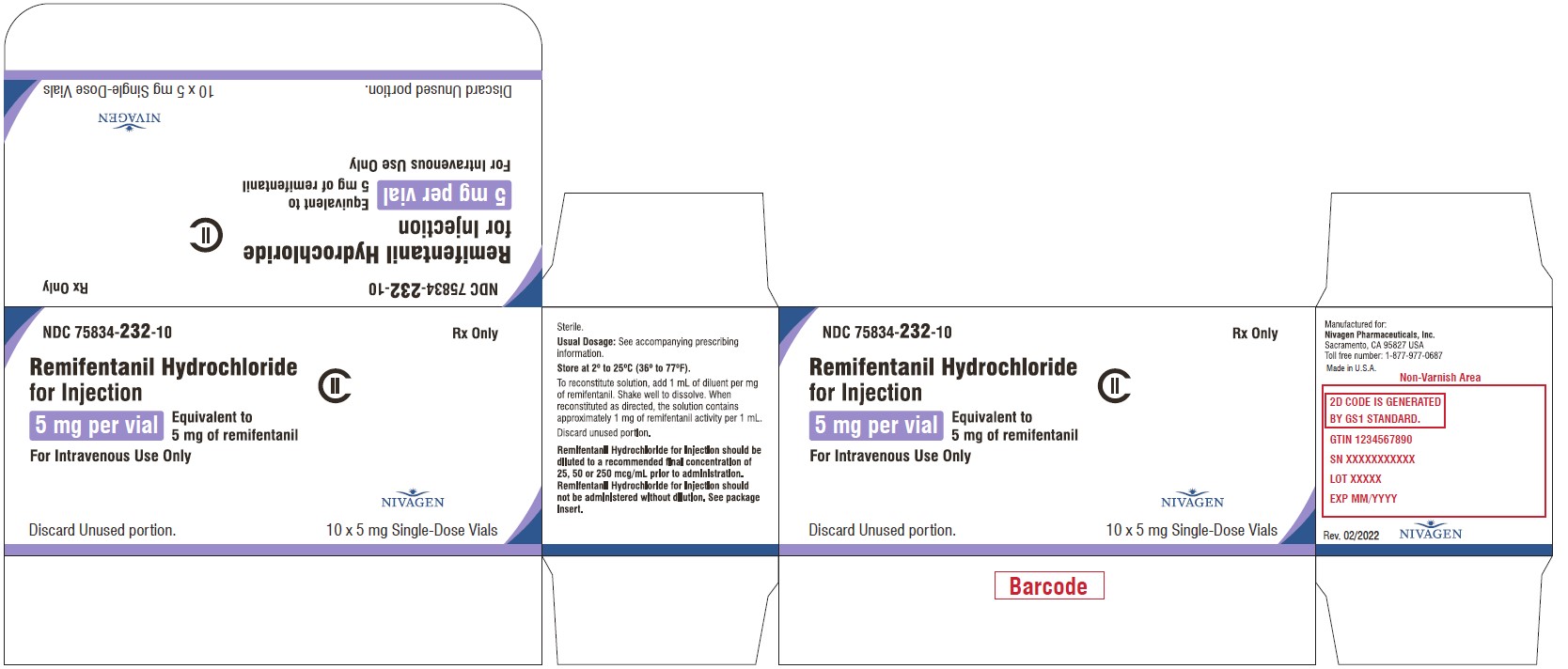
Close -
INGREDIENTS AND APPEARANCEProduct Information
REMIFENTANIL HYDROCHLORIDE remifentanil hydrochloride injection, powder, lyophilized, for solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:75834-230 Route of Administration INTRAVENOUS DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength REMIFENTANIL HYDROCHLORIDE (UNII: 5V444H5WIC) (REMIFENTANIL - UNII:P10582JYYK) REMIFENTANIL 1 mg Inactive Ingredients Ingredient Name Strength GLYCINE (UNII: TE7660XO1C) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75834-230-10 10 in 1 CARTON 09/04/2024 1 NDC:75834-230-01 1 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215635 09/04/2024 REMIFENTANIL HYDROCHLORIDE remifentanil hydrochloride injection, powder, lyophilized, for solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:75834-231 Route of Administration INTRAVENOUS DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength REMIFENTANIL HYDROCHLORIDE (UNII: 5V444H5WIC) (REMIFENTANIL - UNII:P10582JYYK) REMIFENTANIL 2 mg Inactive Ingredients Ingredient Name Strength GLYCINE (UNII: TE7660XO1C) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75834-231-10 10 in 1 CARTON 09/04/2024 1 NDC:75834-231-01 2 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215635 09/04/2024 REMIFENTANIL HYDROCHLORIDE remifentanil hydrochloride injection, powder, lyophilized, for solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:75834-232 Route of Administration INTRAVENOUS DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength REMIFENTANIL HYDROCHLORIDE (UNII: 5V444H5WIC) (REMIFENTANIL - UNII:P10582JYYK) REMIFENTANIL 5 mg Inactive Ingredients Ingredient Name Strength GLYCINE (UNII: TE7660XO1C) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75834-232-10 10 in 1 CARTON 09/04/2024 1 NDC:75834-232-01 5 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215635 09/04/2024
CloseLabeler - Nivagen Pharmaceuticals, Inc. (052032418)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
REMIFENTANIL HYDROCHLORIDE injection, powder, lyophilized, for solution
Number of versions: 1
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Sep 13, 2024 | 4 (current) | download |
RxNorm
REMIFENTANIL HYDROCHLORIDE injection, powder, lyophilized, for solution
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 1729578 | remifentanil 1 MG Injection | PSN |
| 2 | 1729578 | remifentanil 1 MG Injection | SCD |
| 3 | 1729578 | remifentanil (as remifentanil hydrochloride) 1 MG Injection | SY |
| 4 | 1729584 | remifentanil 2 MG Injection | PSN |
| 5 | 1729584 | remifentanil 2 MG Injection | SCD |
| 6 | 1729584 | remifentanil (as remifentanil hydrochloride) 2 MG Injection | SY |
| 7 | 1729710 | remifentanil 5 MG Injection | PSN |
| 8 | 1729710 | remifentanil 5 MG Injection | SCD |
| 9 | 1729710 | remifentanil (as remifentanil hydrochloride) 5 MG Injection | SY |
Get Label RSS Feed for this Drug
REMIFENTANIL HYDROCHLORIDE injection, powder, lyophilized, for solution
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=39284dec-b9bc-4fc8-b2d3-f1ef1323e5d4
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
REMIFENTANIL HYDROCHLORIDE injection, powder, lyophilized, for solution
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 75834-230-01 |
| 2 | 75834-230-10 |
| 3 | 75834-231-01 |
| 4 | 75834-231-10 |
| 5 | 75834-232-01 |
| 6 | 75834-232-10 |






