Label: VERAPAMIL HYDROCHLORIDE injection
- NDC Code(s): 65219-384-01, 65219-384-02
- Packager: FRESENIUS KABI USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
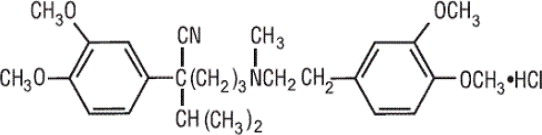
Verapamil hydrochloride is a calcium antagonist or slow-channel inhibitor. Verapamil Hydrochloride Injection, USP is available in 5 mg/2 mL single dose vials (for intravenous administration). Each ...
-
CLINICAL PHARMACOLOGY
Mechanism of Action: Verapamil hydrochloride inhibits the calcium ion (and possibly sodium ion) influx through slow channels into conductile and contractile myocardial cells and vascular ...
-
INDICATIONS AND USAGE
Verapamil hydrochloride injection is indicated for the following: Rapid conversion to sinus rhythm of paroxysmal supraventricular tachycardias, including those associated with accessory bypass ...
-
CONTRAINDICATIONS
Verapamil hydrochloride injection is contraindicated in: Severe hypotension or cardiogenic shock. Second- or third-degree AV block (except in patients with a functioning artificial ventricular ...
-
WARNINGS
VERAPAMIL HYDROCHLORIDE INJECTION SHOULD BE GIVEN AS A SLOW INTRAVENOUS INJECTION OVER AT LEAST A TWO-MINUTE PERIOD OF TIME. (see - DOSAGE AND ADMINISTRATION.) Hypotension: Verapamil ...
-
PRECAUTIONS
Drug Interactions: (see - WARNINGS: Concomitant Antiarrhythmic Therapy) Verapamil hydrochloride injection has been used concomitantly with other cardioactive drugs (especially digitalis) without ...
-
ADVERSE REACTIONS
The following reactions were reported with verapamil hydrochloride injection used in controlled U.S. clinical trials involving 324 patients: Cardiovascular: Symptomatic hypotension (1.5%) ...
-
OVERDOSAGE
Treatment of overdosage should be supportive and individualized. Beta-adrenergic stimulation and/or parenteral administration of calcium solutions may increase calcium ion flux across the slow ...
-
DOSAGE AND ADMINISTRATION (For Intravenous Use Only)
VERAPAMIL HYDROCHLORIDE INJECTION SHOULD BE GIVEN AS A SLOW INTRAVENOUS INJECTION OVER AT LEAST A TWO-MINUTE PERIOD OF TIME UNDER CONTINUOUS ELECTROCARDIOGRAPHIC (ECG) AND BLOOD PRESSURE ...
-
HOW SUPPLIED
Verapamil Hydrochloride Injection, USP, 5 mg per 2 mL (2.5 mg per mL) is a sterile, clear, colorless solution and is supplied in single-dose vials as follows: No ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY – Verapamil Hydrochloride Injection, USP 2 mL Vial Label - NDC 65219-384-01 - Verapamil Hydrochloride Injection, USP - 5 mg per 2 mL - (2.5 mg per mL) For ...
-
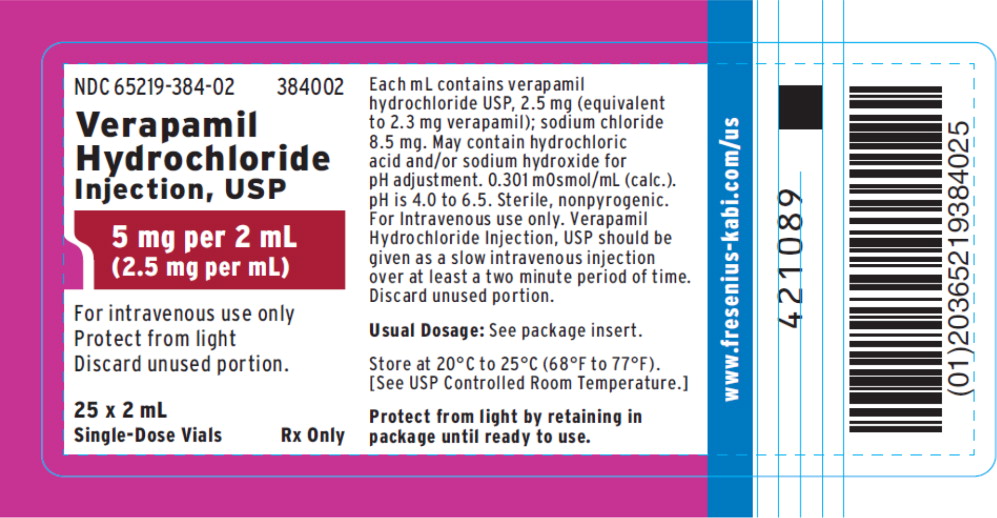
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY – Verapamil Hydrochloride Injection, USP 2 mL Tray Label - NDC 65219-384-02 384002 - Verapamil Hydrochloride Injection, USP - 5 mg per 2 mL - (2.5 mg per ...
-
INGREDIENTS AND APPEARANCEProduct Information