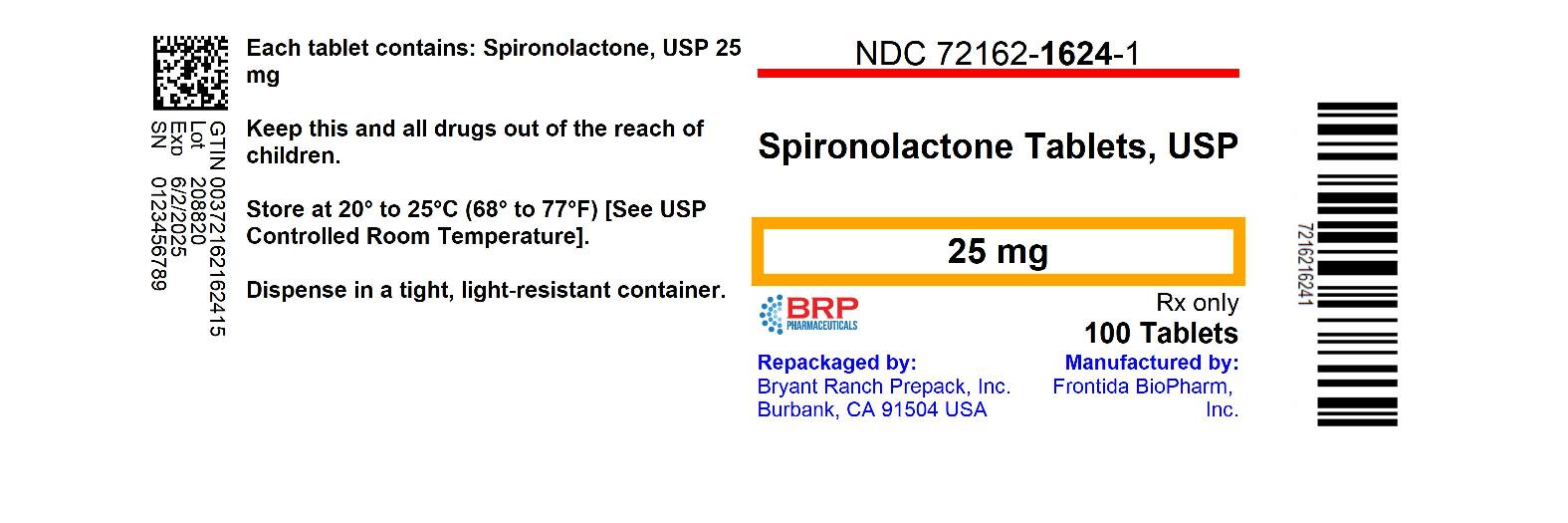
Label: SPIRONOLACTONE tablet
- NDC Code(s): 72162-1624-0, 72162-1624-1, 72162-1624-3, 72162-1624-5, view more
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 53489-143
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 11, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SPIRONOLACTONE TABLETS safely and effectively. See full prescribing information for SPIRONOLACTONE TABLETS. SPIRONOLACTONE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE 1.1 Heart Failure - Spironolactone tablets are indicated for treatment of NYHA Class III-IV heart failure and reduced ejection fraction to increase survival, manage edema, and reduce the need ...
-
2 DOSAGE AND ADMINISTRATION 2.1 General Considerations - Spironolactone tablets can be taken with or without food, but should be taken consistently with respect to food [see Clinical Pharmacology (12.3)] ...
-
3 DOSAGE FORMS AND STRENGTHS Tablets: 25 mg white, round, unscored, debossed MP 35 - Tablets: 50 mg white, round, film coated, scored, debossed MP 542 - Tablets: 100 mg white, oval shape, film coated, scored, debossed MP ...
-
4 CONTRAINDICATIONS Spironolactone tablets are contraindicated in the patients with: • Hyperkalemia • Addison’s disease • Concomitant use of eplerenone
-
5 WARNINGS AND PRECAUTIONS 5.1 Hyperkalemia - Spironolactone tablets can cause hyperkalemia. This risk is increased by impaired renal function or concomitant potassium supplementation, potassium-containing salt ...
-
6 ADVERSE REACTIONS The following clinically significant adverse reactions are described elsewhere in the labeling: • Hyperkalemia [see Warnings and Precautions (5.1)] • Hypotension and Worsening ...
-
7 DRUG INTERACTIONS 7.1 Drugs and Supplements Increasing Serum Potassium - Concomitant administration of spironolactone tablets with potassium supplementation or drugs that can increase potassium may lead to severe ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Based on mechanism of action and findings in animal studies, spironolactone may affect sex differentiation of the male during embryogenesis (see Data). Rat ...
-
10 OVERDOSAGE The oral LD50 of spironolactone tablets is greater than 1000 mg/kg in mice, rats, and rabbits. Acute overdosage of spironolactone tablets may be manifested by drowsiness, mental ...
-
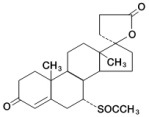
11 DESCRIPTION Spironolactone oral tablets contain 25 mg, 50 mg, or 100 mg of the aldosterone antagonist spironolactone, 17‑ hydroxy-7α-mercapto-3-oxo-17α-pregn-4-ene-21-carboxylic acid γ-lactone acetate, which ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Spironolactone and its active metabolites are specific pharmacologic antagonists of aldosterone, acting primarily through competitive binding of receptors at the ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Orally administered spironolactone tablets has been shown to be a tumorigen in dietary administration studies ...
-
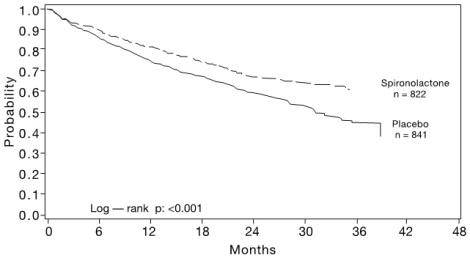
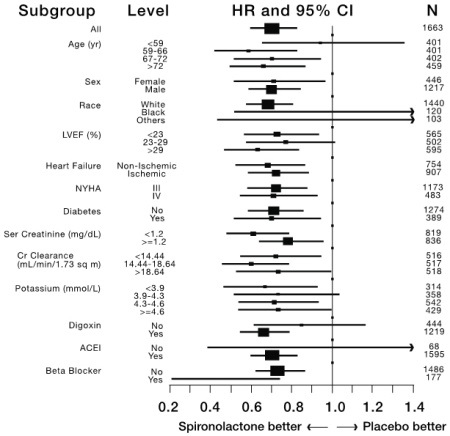
14 CLINICAL STUDIES 14.1 Heart Failure - The Randomized Spironolactone Evaluation Study was a placebo controlled, double-blind study of the effect of spironolactone on mortality in patients with highly symptomatic ...
-
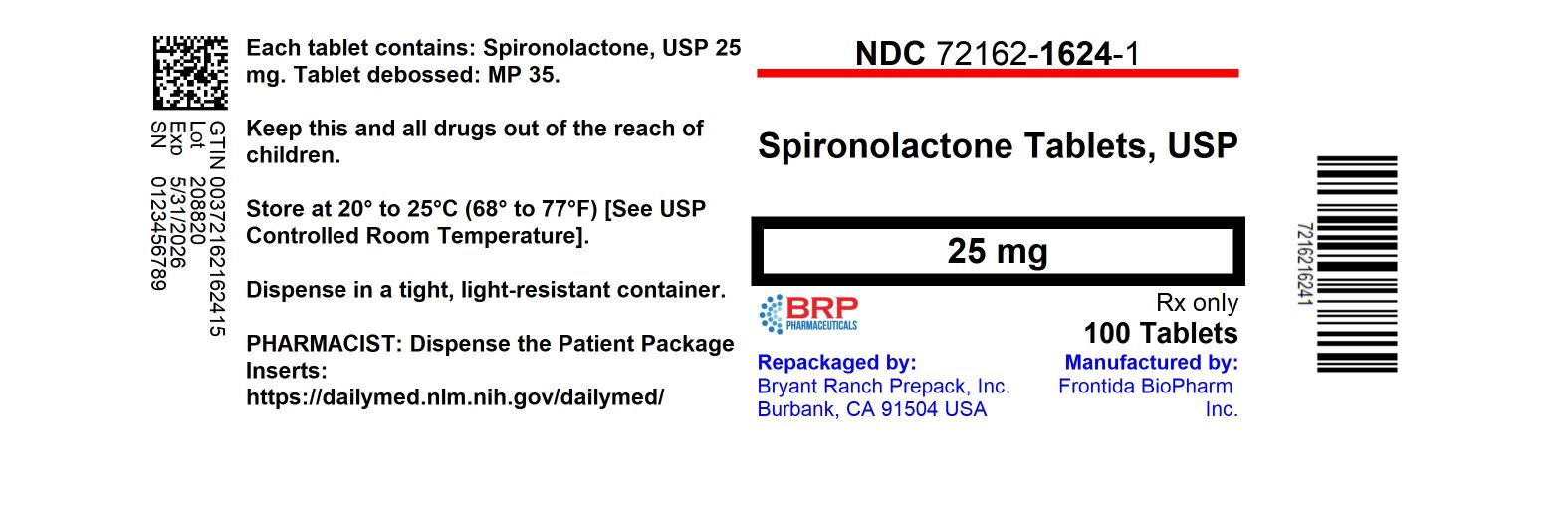
16 HOW SUPPLIED/STORAGE AND HANDLING Spironolactone tablets, USP are supplied as follows: Spironolactone tablets 25 mg, white, round, unscored, debossed MP 35 - NDC 72162-1624-1 Bottles of 100 - NDC 72162-1624-3 Bottles of 30 - NDC ...
-
17 PATIENT COUNSELING INFORMATION Patients who receive spironolactone tablets should be advised to avoid potassium supplements and foods containing high levels of potassium, including salt substitutes. 1. Distributed by: Sun ...
-
PRINCIPAL DISPLAY PANELSPIRONOLACTONE 25 MG TABLET
-
INGREDIENTS AND APPEARANCEProduct Information