Label: ZO SKIN HEALTH PIGMENT CONTROL PROGRAM PLUS HYDROQUINONE- hydroquinone kit
- NDC Code(s): 42851-184-60
- Packager: ZO Skin Health, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 20, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Hydroquinone is 1,4-benzendiol, with a chemical formula of C6H6O2 and a molecular weight of 110.11.
The structural formula is:
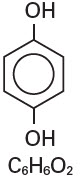
Each gram of Pigment Control Creme (Hydroquinone USP, 4%) contains Hydroquinone USP 40 mg/gm in a base of Purified Water, Ascorbic Acid, Ascorbyl Palmitate, Beta-Glucan, Caprylyl Glycol, Cetyl Alcohol, Chlorphenesin, Dioscorea Villosa (Wild Yam) Root Extract, Disodium EDTA, Glycerin, Glycolic Acid, Phenoxyethanol, Quillaja Saponaria Bark Extract, Smilax Aristolochiifolia Root Extract, Sodium Hydroxide, Sodium Lauryl Sulfate, Sodium Metabisulfite, Sodium Sulfite, Stearyl Alcohol, Tocopheryl Acetate, Yucca Schidigera Root Extract.
-
CLINICAL PHARMACOLOGY
Topical application of hydroquinone produces a reversible depigmentation of the skin by inhibition of the enzymatic oxidation of tyrosine to 3,4-dihydroxyphenylalanine (DOPA) and suppression of other melanocyte metabolic processes. Exposure to sunlight or ultraviolet light will cause re-pigmentation of bleached areas, which may be prevented by the use of the sunscreen agents.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Hydroquinone is a skin bleaching agent which may produce undesired effects if not used as directed. The physician should be familiar with the contents of this insert before prescribing or dispensing this product.
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people.
Avoid unnecessary sun exposure, use an effective broad-spectrum sunscreen agent or protective clothing should be worn to cover bleached skin to prevent re-pigmentation from occurring.
Hydroquinone may produce exogenous ochronosis, a gradual blue-black darkening of the skin. If this condition occurs, discontinue treatment and consult your physician.
Avoid contact with eyes and mucous membranes. Keep out of reach of children. In case of accidental ingestion, call a physician or a poison control center immediately.
-
PRECAUTIONS
Test for skin sensitivity before using by applying a small amount to an unbroken patch of skin; check within 24 hours. Minor redness is not a contraindication, but where there is itching or vesicle formation or excessive inflammatory response, further treatment is not advised. Close patient supervision is recommended.
Drug Interactions
Patients are cautioned on concomitant use of medications that are known to be photosensitizing.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies of hydroquinone in animals have demonstrated some evidence of carcinogenicity. The carcinogenic potential of hydroquinone in humans is unknown.
Pregnancy Category C
Animal reproduction studies have not been conducted with topical hydroquinone. It is also not known whether topical hydroquinone can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Topical hydroquinone should be given to a pregnant woman only if clearly needed.
- Adverse Reactions
- Overdosage
-
DRUG DOSAGE AND ADMINISTRATION
A thin layer of Pigment Control Creme (Hydroquinone USP, 4%) should be applied to the affected area twice daily or as directed by a physician. If no improvement is seen after 8-12 weeks of treatment, use of this product should be discontinued. There is no recommended dosage for pediatric patients under 12 years of age except under the advice and supervision of a physician.
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Hydroquinone is 1,4-benzendiol, with a chemical formula of C6H6O2 and a molecular weight of 110.11.
The structural formula is:
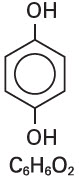
Each gram of Pigment Control + Blending Creme contains Hydroquinone USP 40mg/gm in a base of Ascorbic Acid, Ascorbyl Palmitate, Beta-Glucan, Caprylyl Glycol, Cetyl Alcohol, Chlorphenesin, Dioscorea Villosa (Wild Yam) Root Extract, Disodium EDTA, Ethylhexyl Palmitate, Glycerin, Glycolic Acid, Palmitic Acid, Phenoxyethanol, Phenyl Trimethicone, Purified Water, Quillaja Saponaria Bark Extract, Smilax Aristolochiifolia Root Extract, Sodium Hydroxide, Sodium Lauryl Sulfate, Sodium Metabisulfite, Sodium Sulfite, Stearyl Alcohol, Tocopheryl Acetate, Yucca Schidigera Root Extract.
-
CLINICAL PHARMACOLOGY
Topical application of hydroquinone produces a reversible depigmentation of the skin by inhibition of the enzymatic oxidation of tyrosine to 3,4-dihydroxyphenylalanine (DOPA) and suppression of other melanocyte metabolic processes. Exposure to sunlight or ultraviolet light will cause re-pigmentation of bleached areas, which may be prevented by the use of the sunscreen agents.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Hydroquinone is a skin bleaching agent which may produce undesired effects if not used as directed. The physician should be familiar with the contents of this insert before prescribing or dispensing this product.
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people.
Avoid unnecessary sun exposure, use an effective broad-spectrum sunscreen agent or protective clothing should be worn to cover bleached skin to prevent re-pigmentation from occurring.
Hydroquinone may produce exogenous ochronosis, a gradual blue-black darkening of the skin. If this condition occurs, discontinue treatment and consult your physician.
Avoid contact with eyes and mucous membranes. Keep out of reach of children. In case of accidental ingestion, call a physician or a poison control center immediately.
-
PRECAUTIONS
Test for skin sensitivity before using by applying a small amount to an unbroken patch of skin; check within 24 hours. Minor redness is not a contraindication, but where there is itching or vesicle formation or excessive inflammatory response, further treatment is not advised. Close patient supervision is recommended.
Drug Interactions
Patients are cautioned on concomitant use of medications that are known to be photosensitizing.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies of hydroquinone in animals have demonstrated some evidence of carcinogenicity. The carcinogenic potential of hydroquinone in humans is unknown.
Pregnancy Category C
Animal reproduction studies have not been conducted with topical hydroquinone. It is also not known whether topical hydroquinone can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Topical hydroquinone should be given to a pregnant woman only if clearly needed.
- Adverse Reactions
- Overdosage
-
DRUG DOSAGE AND ADMINISTRATION
A thin application of Pigment Control + Blending Creme should be applied to the affected area twice daily or as directed by a physician. Consult product label for instructions on whether to rub in or not. There is no recommendation for children under 12 years of age except under the advice and supervision of a physician.
- HOW SUPPLIED
-
PRINCIPAL DISPLAY PANEL - Kit Carton
ZO ® SKIN HEALTH
BY ZEIN OBAGI MDPIGMENT CONTROL PROGRAM
+ HYDROQUINONENDC 42851-184-60
GENTLE CLEANSER 60 mL / 2 Fl. Oz.
EXFOLIATING POLISH Net Wt. 16.2 g / 0.57 Oz.
COMPLEXION RENEWAL PADS 30 Pads
PIGMENT CONTROL CRÈME 30 mL / 1.0 Fl. Oz.
DAILY POWER DEFENSE 30 mL / 1 Fl. Oz.
PIGMENT CONTROL + BLENDING CREME 30 mL / 1 Fl. Oz.

-
INGREDIENTS AND APPEARANCE
ZO SKIN HEALTH PIGMENT CONTROL PROGRAM PLUS HYDROQUINONE
hydroquinone kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42851-184 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42851-184-60 1 in 1 CARTON 03/09/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 60 mL Part 2 1 JAR 16.2 g Part 3 30 JAR 30 Part 4 1 BOTTLE, PLASTIC 30 mL Part 5 1 BOTTLE, PLASTIC 30 mL Part 6 1 BOTTLE, PUMP 30 mL Part 1 of 6 ZO SKIN HEALTH GENTLE CLEANSER
cleansing (cold creams, cleansing lotions, liquids, and pads) gelProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) INGR COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) INGR SODIUM LAUROYL OAT AMINO ACIDS (UNII: FSW2K9B9N5) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR GREEN TEA LEAF (UNII: W2ZU1RY8B0) INGR LIMONENE, (+/-)- (UNII: 9MC3I34447) INGR LINALOOL, (+/-)- (UNII: D81QY6I88E) INGR SODIUM CHLORIDE (UNII: 451W47IQ8X) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR FD&C BLUE NO. 1 (UNII: H3R47K3TBD) INGR D&C RED NO. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 60 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 03/09/2021 Part 2 of 6 ZO SKIN HEALTH EXFOLIATING POLISH
lotions, oils, powders, and creams suspensionProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) INGR SODIUM BICARBONATE (UNII: 8MDF5V39QO) INGR MAGNESIUM OXIDE (UNII: 3A3U0GI71G) INGR DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR OLETH-20 (UNII: YTH167I2AG) INGR TRIHYDROXYSTEARIN (UNII: 06YD7896S3) INGR GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) INGR PEG-100 STEARATE (UNII: YD01N1999R) INGR MINERAL OIL (UNII: T5L8T28FGP) INGR WATER (UNII: 059QF0KO0R) INGR .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) INGR TEA TREE OIL (UNII: VIF565UC2G) INGR SOY STEROL (UNII: PL360EPO9J) INGR LIMONENE, (+/-)- (UNII: 9MC3I34447) INGR LINALOOL, (+/-)- (UNII: D81QY6I88E) INGR ASCORBYL PALMITATE (UNII: QN83US2B0N) INGR VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) INGR STEARYL GLYCYRRHETINATE (UNII: 3YYE6VJS0P) INGR TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR LINOLEIC ACID (UNII: 9KJL21T0QJ) INGR LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR D&C GREEN NO. 6 (UNII: 4QP5U84YF7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 16.2 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 03/09/2021 Part 3 of 6 ZO SKIN HEALTH COMPLEXION RENEWAL PADS
cleansing (cold creams, cleansing lotions, liquids, and pads) patchProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR ALCOHOL (UNII: 3K9958V90M) INGR SALICYLIC ACID (UNII: O414PZ4LPZ) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR GLYCOLIC ACID (UNII: 0WT12SX38S) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR SODIUM CARBONATE (UNII: 45P3261C7T) INGR EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) INGR DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) INGR .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) INGR .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) INGR LINALOOL, (+/-)- (UNII: D81QY6I88E) INGR UREA (UNII: 8W8T17847W) INGR BARLEY (UNII: 5PWM7YLI7R) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR SODIUM CHLORIDE (UNII: 451W47IQ8X) INGR TERT-BUTYL ALCOHOL (UNII: MD83SFE959) INGR PTEROCARPUS SOYAUXII WOOD (UNII: 0V6QB4C61P) INGR GREEN TEA LEAF (UNII: W2ZU1RY8B0) INGR LIMONENE, (+)- (UNII: GFD7C86Q1W) INGR WATER (UNII: 059QF0KO0R) INGR PROPYLENE GLYCOL (UNII: 6DC9Q167V3) INGR PLANTAGO LANCEOLATA LEAF (UNII: 2YWL9J7EE8) INGR PHELLODENDRON AMURENSE BARK (UNII: PBG27B754G) INGR CRITHMUM MARITIMUM (UNII: J7IHY79BKY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 03/09/2021 Part 4 of 6 ZO SKIN HEALTH PIGMENT CONTROL CREME HYDROQUINONE
hydroquinone emulsionProduct Information Item Code (Source) NDC:42851-037 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROQUINONE (UNII: XV74C1N1AE) (HYDROQUINONE - UNII:XV74C1N1AE) HYDROQUINONE 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ASCORBIC ACID (UNII: PQ6CK8PD0R) ASCORBYL PALMITATE (UNII: QN83US2B0N) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CETYL ALCOHOL (UNII: 936JST6JCN) CHLORPHENESIN (UNII: I670DAL4SZ) DIOSCOREA VILLOSA TUBER (UNII: IWY3IWX2G8) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) GLYCERIN (UNII: PDC6A3C0OX) GLYCOLIC ACID (UNII: 0WT12SX38S) PHENOXYETHANOL (UNII: HIE492ZZ3T) QUILLAJA SAPONARIA BARK (UNII: 8N0K3807ZW) SMILAX ARISTOLOCHIIFOLIA ROOT (UNII: NR100Y25G0) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM METABISULFITE (UNII: 4VON5FNS3C) SODIUM SULFITE (UNII: VTK01UQK3G) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) YUCCA SCHIDIGERA ROOT (UNII: E2H9ET15AT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/09/2021 Part 5 of 6 ZO SKIN HEALTH PIGMENT CONTROL PLUS BLENDING CREME HYDROQUINONE
hydroquinone emulsionProduct Information Item Code (Source) NDC:42851-036 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROQUINONE (UNII: XV74C1N1AE) (HYDROQUINONE - UNII:XV74C1N1AE) HYDROQUINONE 0.04 g in 1 mL Inactive Ingredients Ingredient Name Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) ASCORBYL PALMITATE (UNII: QN83US2B0N) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CETYL ALCOHOL (UNII: 936JST6JCN) CHLORPHENESIN (UNII: I670DAL4SZ) DIOSCOREA VILLOSA TUBER (UNII: IWY3IWX2G8) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) ETHYLHEXYL PALMITATE (UNII: 2865993309) GLYCERIN (UNII: PDC6A3C0OX) GLYCOLIC ACID (UNII: 0WT12SX38S) PALMITIC ACID (UNII: 2V16EO95H1) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) QUILLAJA SAPONARIA BARK (UNII: 8N0K3807ZW) SMILAX ARISTOLOCHIIFOLIA ROOT (UNII: NR100Y25G0) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM METABISULFITE (UNII: 4VON5FNS3C) SODIUM SULFITE (UNII: VTK01UQK3G) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) WATER (UNII: 059QF0KO0R) YUCCA SCHIDIGERA ROOT (UNII: E2H9ET15AT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/09/2021 Part 6 of 6 ZO SKIN HEALTH DAILY POWER DEFENSE
other skin care preparations lotionProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR PENTYLENE GLYCOL (UNII: 50C1307PZG) INGR POWDERED CELLULOSE (UNII: SMD1X3XO9M) INGR RETINOL (UNII: G2SH0XKK91) INGR LIMONENE, (+)- (UNII: GFD7C86Q1W) INGR CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) INGR C12-20 ALKYL GLUCOSIDE (UNII: K67N5Z1RUA) INGR LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) INGR PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) INGR PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR STEARETH-20 (UNII: L0Q8IK9E08) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR CETEARYL ISONONANOATE (UNII: P5O01U99NI) INGR CYCLOMETHICONE 6 (UNII: XHK3U310BA) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR EXT. D&C VIOLET NO. 2 (UNII: G5UX3K0728) INGR EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) INGR HEXYLENE GLYCOL (UNII: KEH0A3F75J) INGR ARABIDOPSIS THALIANA (UNII: AI3L60HQ81) INGR ULTRAMARINE BLUE (UNII: I39WR998BI) INGR 1,2-HEXANEDIOL (UNII: TR046Y3K1G) INGR C14-22 ALCOHOLS (UNII: B1K89384RJ) INGR CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) INGR CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) INGR WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 03/09/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/09/2021 Labeler - ZO Skin Health, Inc. (826468527)

