Label: HYOSCYAMINE SULFATE SL- hyoscyamine sulfate tablet
-
NDC Code(s):
71335-1159-0,
71335-1159-1,
71335-1159-2,
71335-1159-3, view more71335-1159-4, 71335-1159-5, 71335-1159-6, 71335-1159-7, 71335-1159-8, 71335-1159-9
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 42192-339
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
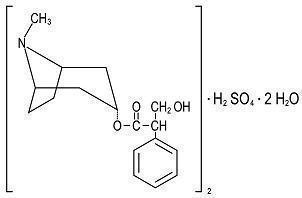
- DESCRIPTION
- INACTIVE INGREDIENTS
-
CLINICAL PHARMACOLOGY
Hyoscyamine has actions similar to those of atropine, but is more potent in both its central and peripheral effects.
This product inhibits gastrointestinal propulsive motility and decreases gastric acid secretions. This product controls excessive pharyngeal, tracheal, and bronchial secretion. This product is absorbed totally and completely by sublingual administration as well as oral administration.
Once absorbed, this product disappears rapidly from the blood and is distributed throughout the entire body.
The majority of hyoscyamine sulfate is excreted in the urine unchanged within the first 12 hours and only traces of hyoscyamine sulfate are found in the breast milk.
-
INDICATIONS AND USAGE
This product may be used in functional intestinal disorders to reduce symptoms such as those seen in mild dysenteries and diverticulitis. It can also be used to control gastric secretion, visceral spasm and hypermotility in cystitis, pylorospasm and associated abdominal cramps.
Along with appropriate analgesics, this product is indicated in symptomatic relief of biliary and renal colic and as a drying agent in the relief of symptoms of acute rhinitis.
This product is effective as adjunctive therapy in the treatment of peptic ulcer and irritable bowel syndrome, acute enterocolitis and other functional gastrointestinal disorders.
-
CONTRAINDICATIONS
Glaucoma, obstructive uropathy, obstructive diseases of the gastrointestinal tract, paralytic ileum, intestinal atony of elderly or debilitated patients, unstable cardiovascular status, severe ulcerative colitis, toxic megacolon, myasthenia gravis, and myocardial ischemia. This product is not recommended for use in children under twelve years of age.
-
WARNINGS
Heat prostration can occur with drug use in the event of high environmental temperature.
Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy; in this instance, treatment would be inappropriate and possibly harmful. This product may cause drowsiness or blurred vision. Patients taking this product should be warned not to engage in activities requiring mental alertness such as operating a motor vehicle or other machinery or to perform hazardous tasks while taking this drug.
-
PRECAUTIONS
Use caution in patients with hiatal hernia associated with reflex esophagitis. Use extreme caution and only when needed in patients with autonomic neuropathy, hyperthyroidism, coronary heart disease, congestive heart failure and cardiac arrhythmia. Investigate any tachycardia before giving any anticholinergic drugs since they may increase the heart rate.
Prolonged use of anticholinergics may decrease or inhibit salivary flow, thus contributing to the development of caries, periodontal disease, oral candidiasis, and discomfort.
Information for Patients:
This medication should be taken 30 minutes to one hour before meals. This medication should be used with caution during exercise or hot weather; overheating may result in heat stroke. Hyoscyamine may cause drowsiness, dizziness or blurred vision; patients should observe caution before driving, using machinery or performing other tasks requiring mental alertness.
Drug Interactions:
Absorption of other oral medications may be decreased during concurrent use with anticholinergics due to decreased gastrointestinal motility and delayed gastric emptying. Drug interactions may occur when anticholinergics are used with the following medications: antacids, antidiarrheals (adsorbent), other anticholinergics, antimyasthenics, cyclopropane, haloperidol, ketoconazole, metoclopramide, opioid (narcotic) analgesics, and potassium chloride.
Pregnancy:
Pregnancy Category C.Animal reproduction studies have not been conducted with this product. It is also not known whether this product can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity.
Hyoscyamine crosses the placenta. This product should be given to a pregnant woman only if clearly needed.
Nursing Mothers:
This product is excreted in human milk. This product should not be administered to a nursing mother.
Pediatric Use:
This product is not recommended for use in children under twelve years of age. Infants and young children are especially susceptible to the toxic effects of anticholinergics. Close supervision is recommended for infants and children with spastic paralysis or brain damage since an increased response to anticholinergics has been reported in these patients and dosage adjustments are often required.
When anticholinergics are given to children where the environmental temperature is high, there is a risk of a rapid increase in body temperature because of these medications’ suppression of sweat gland activity. A paradoxical reaction characterized by hyperexcitability may occur in children taking large doses of anticholinergics.
Geriatric Use:
Geriatric patients may respond to usual doses of anticholinergics with excitement, agitation, drowsiness, or confusion.
Geriatric patients are especially susceptible to the anticholinergic side effects, such as constipation, dryness of mouth, and urinary retention (especially in males). If these side effects occur and continue or are severe, medication should probably be discontinued. Caution is also recommended when anticholinergics are given to geriatric patients, because of the danger of precipitating undiagnosed glaucoma.
Memory may become severely impaired in geriatric patients, especially those who already have memory problems, with the continued use of anticholinergics since these drugs block the actions of acetylcholine, which is responsible for many functions of the brain, including memory functions.
-
ADVERSE REACTIONS
Not all of the following adverse reactions have been reported with hyoscyamine sulfate. The following adverse reactions have been reported for pharmacologically similar drugs with anticholinergic/ antispasmodic action. Adverse reactions may include dryness of the mouth, urinary hesitancy and retention; blurred vision; tachycardia; palpitations; mydriasis; cycloplegia; increased ocular tension; loss of taste; headache; nervousness; drowsiness; weakness; dizziness; insomnia; nausea; vomiting; impotence; suppression of lactation; constipation; bloated feeling; allergic reactions or drug idiosyncrasies; urticaria and other dermal manifestations; ataxia; speech disturbance; some degree of mental confusion and/or excitement (especially in elderly persons); and decreased sweating.
-
OVERDOSAGE
The signs and symptoms of overdose are headache, nausea, vomiting, blurred vision, dilated pupils, hot dry skin, dizziness, dryness of the mouth, difficulty in swallowing and CNS stimulation. Measures to be taken are immediate lavage of the stomach and injection of physostigmine 0.5 to 2 mg intravenously and repeated as necessary up to a total of 5 mg. Fever may be treated symptomatically (tepid water sponge baths, hypothermic blanket). Excitement to a degree which demands attention may be managed with sodium thiopental 2% solution given slowly intravenously or chloral hydrate (100- 200 mL of a 2% solution) by rectal infusion.
In the event of progression of the curare-like effect to paralysis of the respiratory muscles, artificial respiration should be instituted and maintained until effective respiratory action returns.
In rats, the LD 50for hyoscyamine is 375 mg/kg. Hyoscyamine is dialyzable.
-
DOSAGE AND ADMINISTRATION
Dosage may be adjusted according to the condition and severity of symptoms. May be taken with or without water.
Adults and adolescents 12 years of age and older:1 to 2 tablets sublingually three or four times a day, thirty minutes to one hour before meals and at bedtime. Dosage may be increased to every four hours as needed. Do not exceed 12 tablets in 24 hours.
Note:Geriatric patients may be more sensitive to the effects of the usual adult dose.
-
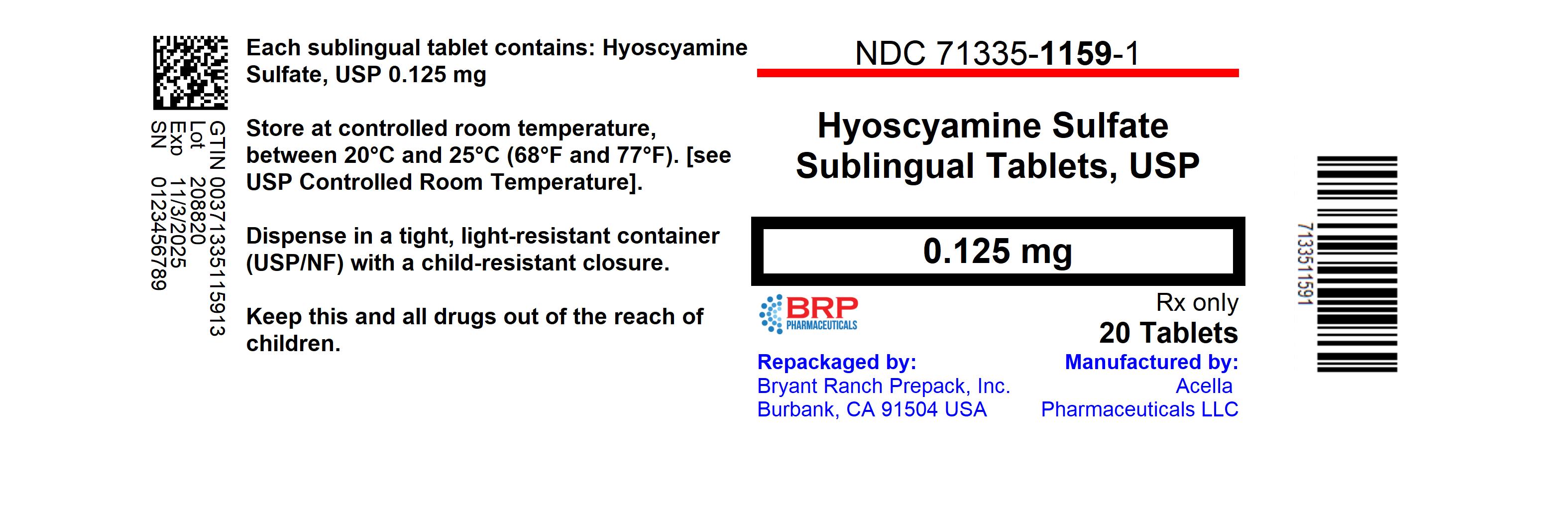
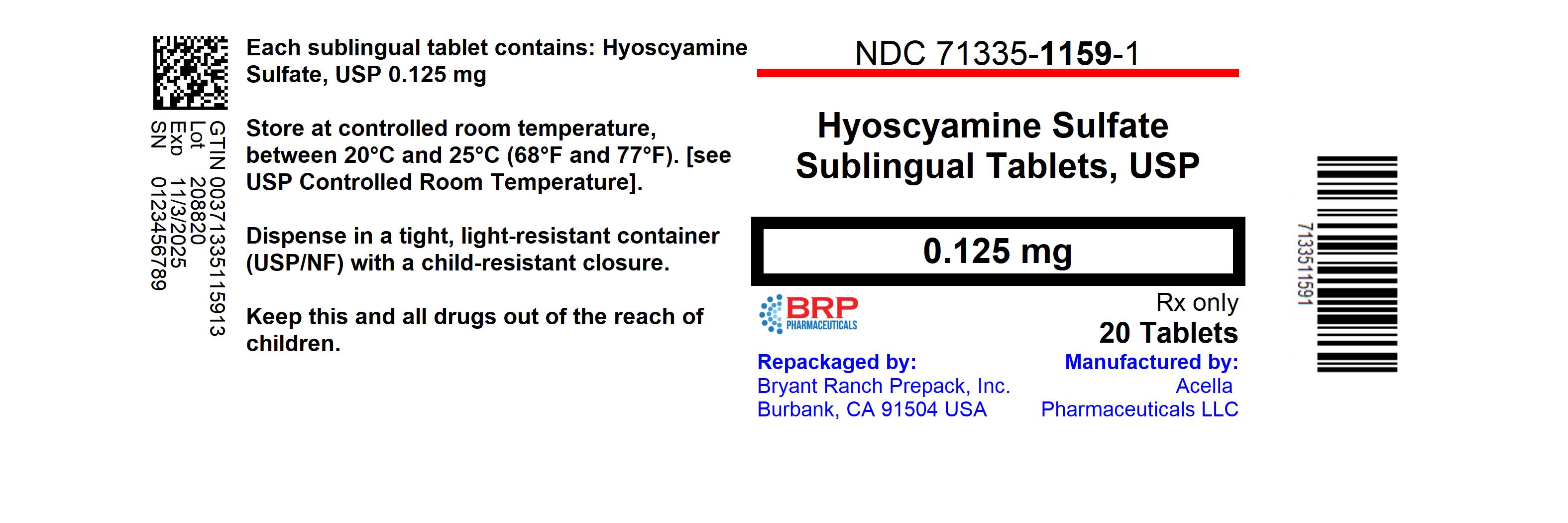
HOW SUPPLIED
Hyoscyamine Sulfate SL is supplied as round, green, peppermint flavored tablets with "39" debossed on one side.
- NDC: 71335-1159-1: 20 Tablets in a BOTTLE
- NDC: 71335-1159-2: 30 Tablets in a BOTTLE
- NDC: 71335-1159-3: 15 Tablets in a BOTTLE
- NDC: 71335-1159-4: 21 Tablets in a BOTTLE
- NDC: 71335-1159-5: 60 Tablets in a BOTTLE
- NDC: 71335-1159-6: 12 Tablets in a BOTTLE
- NDC: 71335-1159-7: 90 Tablets in a BOTTLE
- NDC: 71335-1159-8: 100 Tablets in a BOTTLE
- NDC: 71335-1159-9: 45 Tablets in a BOTTLE
- NDC: 71335-1159-0: 10 Tablets in a BOTTLE
Storage and Handling
Dispense in a tight, light-resistant container as defined in USP/NF, with a child-resistant closure.Store at controlled room temperature between 20°-25°C (68°-77°F), see USP Controlled Room Temperature.
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HYOSCYAMINE SULFATE SL
hyoscyamine sulfate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71335-1159(NDC:42192-339) Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYOSCYAMINE SULFATE (UNII: F2R8V82B84) (HYOSCYAMINE - UNII:PX44XO846X) HYOSCYAMINE SULFATE 0.125 mg Inactive Ingredients Ingredient Name Strength D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color green Score no score Shape ROUND Size 6mm Flavor PEPPERMINT Imprint Code 39 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71335-1159-0 10 in 1 BOTTLE; Type 0: Not a Combination Product 10/31/2024 2 NDC:71335-1159-1 20 in 1 BOTTLE; Type 0: Not a Combination Product 04/20/2020 3 NDC:71335-1159-2 30 in 1 BOTTLE; Type 0: Not a Combination Product 03/19/2019 4 NDC:71335-1159-3 15 in 1 BOTTLE; Type 0: Not a Combination Product 10/31/2024 5 NDC:71335-1159-4 21 in 1 BOTTLE; Type 0: Not a Combination Product 10/31/2024 6 NDC:71335-1159-5 60 in 1 BOTTLE; Type 0: Not a Combination Product 10/31/2024 7 NDC:71335-1159-6 12 in 1 BOTTLE; Type 0: Not a Combination Product 08/02/2024 8 NDC:71335-1159-7 90 in 1 BOTTLE; Type 0: Not a Combination Product 12/13/2021 9 NDC:71335-1159-8 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/04/2022 10 NDC:71335-1159-9 45 in 1 BOTTLE; Type 0: Not a Combination Product 10/31/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/19/2011 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(71335-1159) , RELABEL(71335-1159)