Label: PREHEVBRIO (hepatitis b vaccine- recombinant injection, suspension
- NDC Code(s): 75052-001-01, 75052-001-10
- Packager: VBI Vaccines (Delaware) Inc.
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PREHEVBRIO - TM safely and effectively. See full prescribing information for PREHEVBRIO. PREHEVBRIO [Hepatitis B Vaccine ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
PREHEVBRIO is indicated for prevention of infection caused by all known subtypes of hepatitis B virus. PREHEVBRIO is approved for use in adults 18 years of age and older.
-
2 DOSAGE AND ADMINISTRATION
For intramuscular injection. 2.1 Dosage and Schedule - Administer a series of three doses (1.0 mL each) of PREHEVBRIO on a 0-, 1- and 6-month schedule. 2.2 Administration - Shake the vial ...
-
3 DOSAGE FORMS AND STRENGTHS
PREHEVBRIO is an injectable suspension, for intramuscular use supplied as a single-dose vial. A single dose of PREHEVBRIO is 1.0 mL [see - How Supplied/Storage and Handling ...
-
4 CONTRAINDICATIONS
Do not administer PREHEVBRIO to individuals with a history of severe allergic reaction (e.g., anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of PREHEVBRIO [see ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Managing Allergic Reactions - Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of PREHEVBRIO. 5.2 ...
-
6 ADVERSE REACTIONS
Individuals 18 through 44 years of age: The most common local reactions following each dose of PREHEVBRIO were injection site pain (52.0 – 58.3%) and tenderness (52.6 – 59.6%). The most common ...
-
7 DRUG INTERACTIONS
7.1 Concomitant Administration with Immune Globulin - There are no data to assess the concomitant use of PREHEVBRIO with immune globulin. When concomitant administration of PREHEVBRIO and immune ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to PREHEVBRIO during pregnancy. Women who receive ...
-
11 DESCRIPTION
PREHEVBRIO [Hepatitis B Vaccine (Recombinant)] is a sterile suspension for intramuscular injection. PREHEVBRIO contains the small (S), middle (pre-S2) and large (pre-S1) hepatitis B surface ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - PREHEVBRIO induces antibodies to HBsAg. Antibody concentrations ≥10 mIU/mL against HBsAg are recognized as conferring protection against hepatitis B virus ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - PREHEVBRIO has not been evaluated for carcinogenic, mutagenic potential or male infertility in animals. In a developmental toxicity ...
-
14 CLINICAL STUDIES
14.1 Evaluation of Immunogenicity - The immunogenicity of PREHEVBRIO was evaluated in comparison with a US-licensed hepatitis B vaccine (Engerix-B) in 2 randomized, active controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied - Single dose vial, 1.0 mL (NDC number 75052-001-01) Supplied as a package of 10 single dose vials (NDC number: 75052-001-10) The vial stoppers are not made with natural ...
-
17 PATIENT COUNSELING INFORMATION
Inform vaccine recipient of the potential benefits and risks associated with vaccination with PREHEVBRIO, as well as the importance of completing the immunization series. Emphasize that ...
-
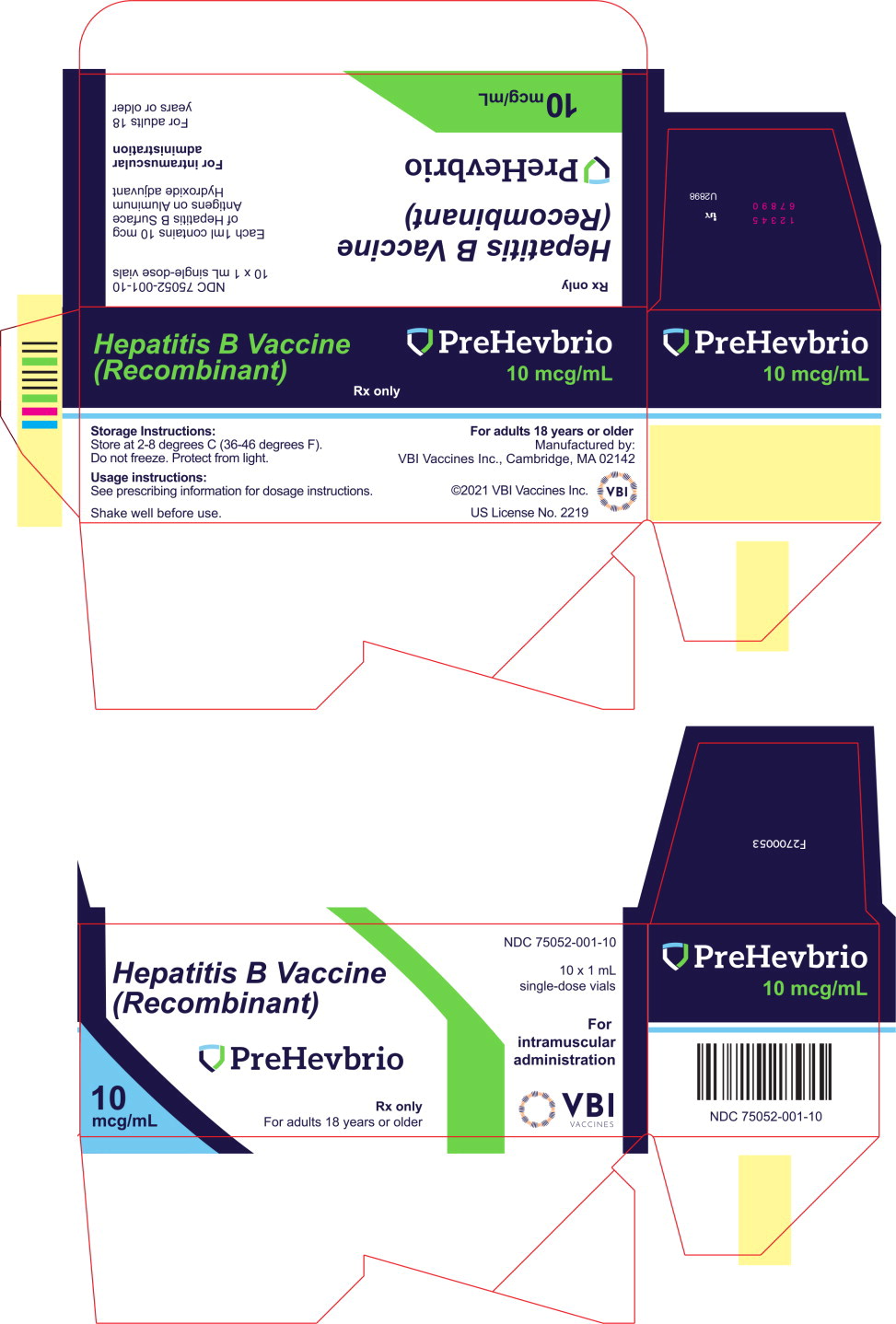
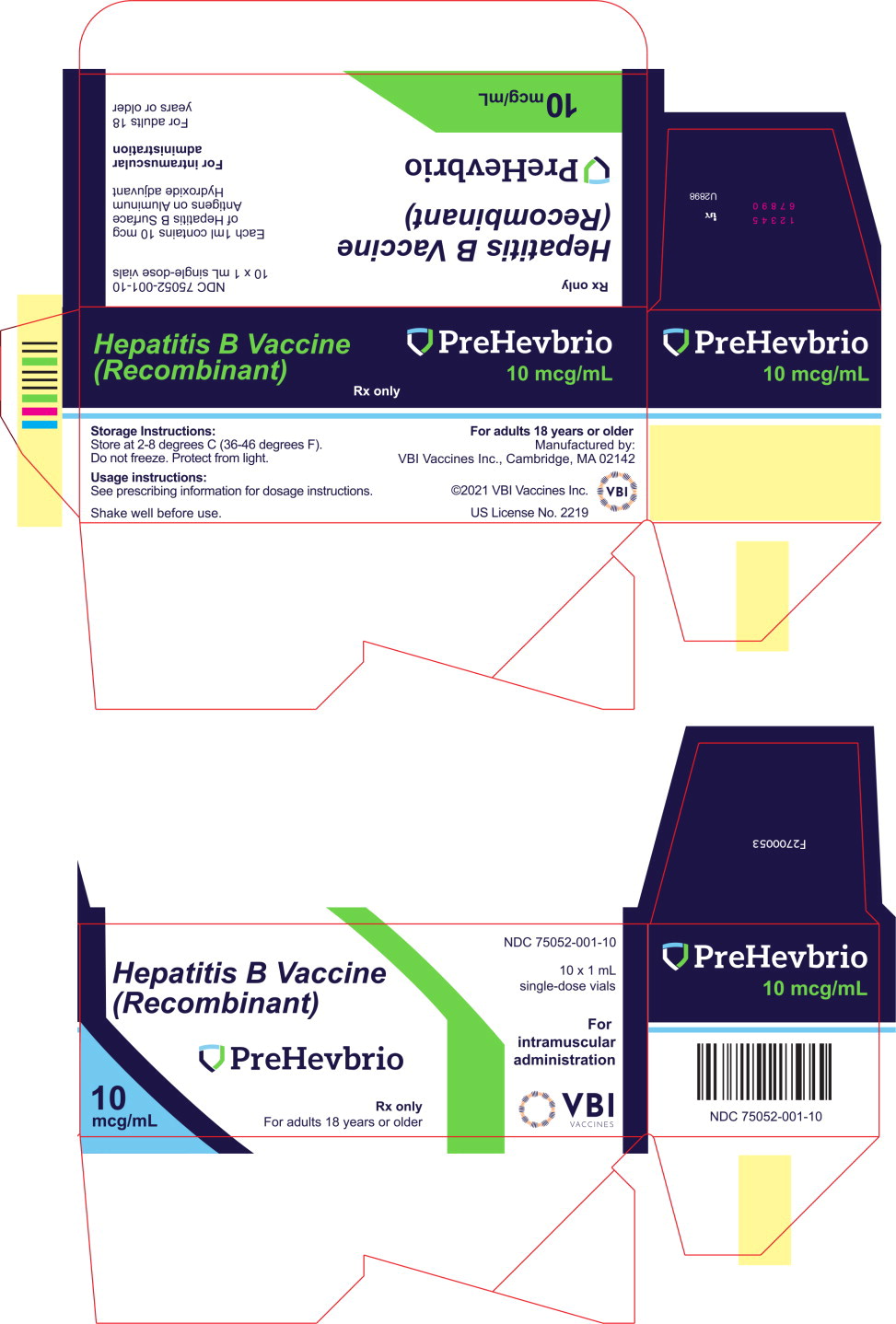
PRINCIPAL DISPLAY PANELPrincipal Display Panel - 10 mcg/mL Carton Label - NDC 75052-001-10 - Hepatitis B Vaccine - (Recombinant) PreHevbrio - Rx only - For adults 18 years or older - 10 ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - 10 mcg/mL Vial Label - NDC 75052-001-01 - Hepatitis B Vaccine - (Recombinant) PreHevbrio - For IM administration - Rx only - Storage ...
-
INGREDIENTS AND APPEARANCEProduct Information