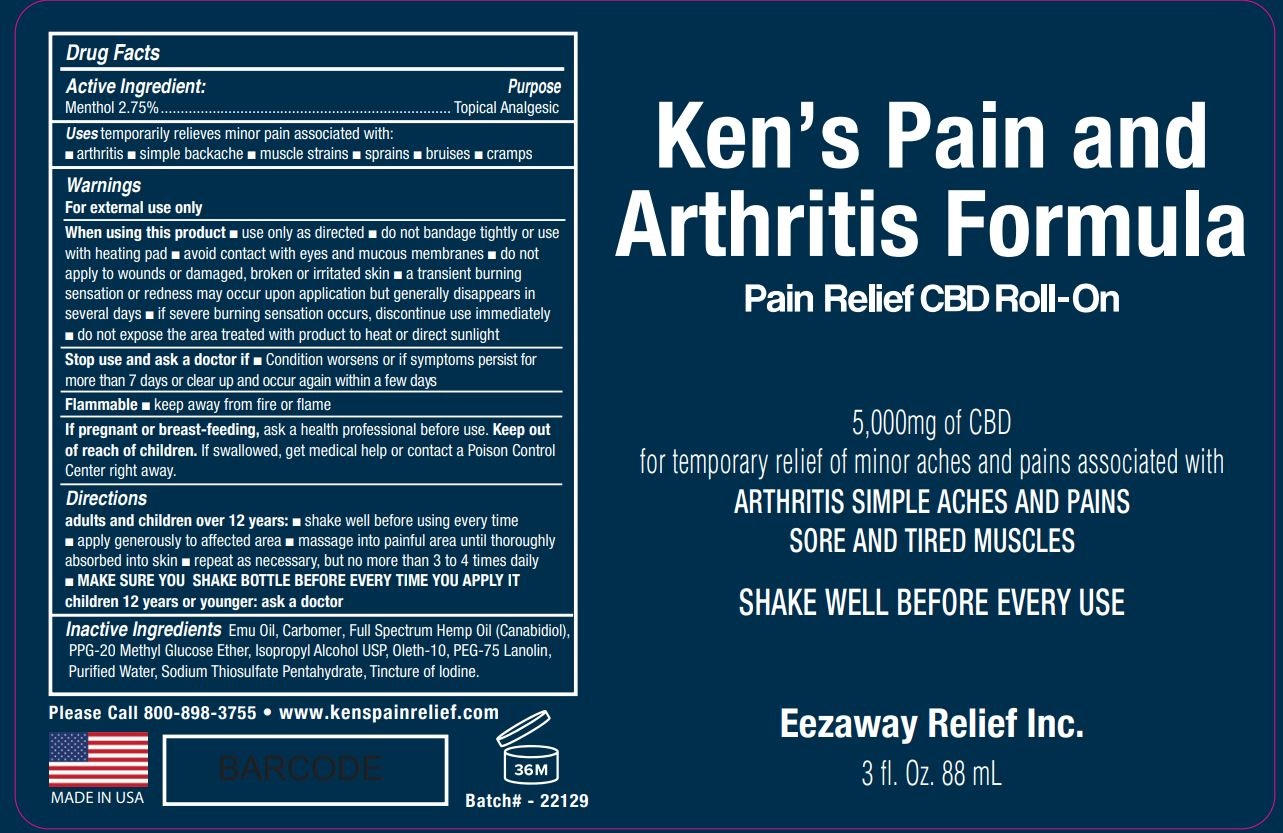
Label: KENS PAIN AND ARTHRITIS FORMULA- menthol liquid
- NDC Code(s): 69678-143-03
- Packager: EEZAWAY RELIEF INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 21, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient:
- Purpose
- Uses
-
Warnings
For external use only
When using this product • use only as directed • do not bandage tightly or use with heating pad • avoid contact with eyes and mucous membranes • do not apply to wounds or damaged, broken or irritated skin • a transient burning sensation or redness may occur upon application but generally disappears in several days • if severe burning sensation occurs, discontinue use immediately • do not expose the area treated with product to heat or direct sunlight
Stop use and ask a doctor if • Condition worsens or if symptoms persist for more than 7 days or clear up and occur again within a few days
Flammable • keep away from fire or flame
If pregnant or breast-feeding, ask a health professional before use.
-
Directions
adults and children over 12 years: • shake well before using every time • apply generously to affected area • massage into painful area until thoroughly absorbed into skin • repeat as necessary, but no more than 3 to 4 times daily
• MAKE SURE YOU SHAKE BOTTLE BEFORE EVERY TIME YOU APPLY IT
children 12 years or younger: ask a doctor
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
KENS PAIN AND ARTHRITIS FORMULA
menthol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69678-143 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 2.75 g in 100 mL Inactive Ingredients Ingredient Name Strength EMU OIL (UNII: 344821WD61) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) PPG-20 METHYL GLUCOSE ETHER (UNII: 3WV1T97D3K) ISOPROPYL ALCOHOL (UNII: ND2M416302) OLETH-10 (UNII: JD797EF70J) PEG-75 LANOLIN (UNII: 09179OX7TB) WATER (UNII: 059QF0KO0R) SODIUM THIOSULFATE (UNII: HX1032V43M) IODINE (UNII: 9679TC07X4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69678-143-03 88 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/05/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/05/2024 Labeler - EEZAWAY RELIEF INC (109456638)