Label: GENERLAC- lactulose solution
- NDC Code(s): 62135-892-47, 62135-892-94
- Packager: Chartwell RX, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
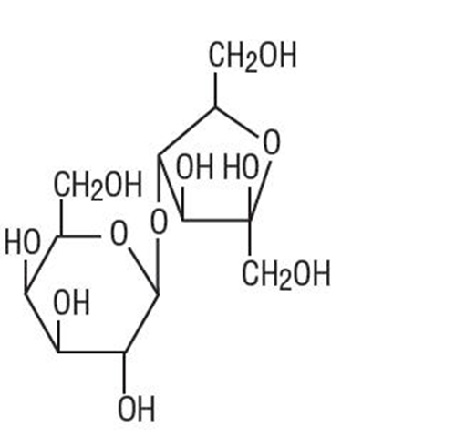
DESCRIPTIONGenerlac Solution (Lactulose Solution, USP) is a synthetic disaccharide in solution form for oral or rectal administration. Each 15 mL of Generlac Solution contains:10 g lactulose (and less ...
-
CLINICAL PHARMACOLOGYLactulose causes a decrease in blood ammonia concentration and reduces the degree of portal-systemic encephalopathy. These actions are considered to be results of the following: Bacterial ...
-
INDICATIONS AND USAGEFor the prevention and treatment of portal-systemic encephalopathy, including the stages of hepatic pre-coma and coma. Controlled studies have shown that lactulose solution therapy reduces the ...
-
CONTRAINDICATIONSSince Generlac Solution (Lactulose Solution, USP) contains galactose (less than 1.6 g/15 mL), it is contraindicated in patients who require a low galactose diet.
-
WARNINGSA theoretical hazard may exist for patients being treated with lactulose solution who may be required to undergo electrocautery procedures during proctoscopy or colonoscopy. Accumulation of H ...
-
PRECAUTIONSGeneral - Since Generlac Solution contains galactose (less than 1.6 g/15 mL) and lactose (less than 1.2 g/15 mL), it should be used with caution in diabetics. In the overall management of ...
-
ADVERSE REACTIONSPrecise frequency data are not available. Generlac Solution may produce gaseous distention with flatulence or belching and abdominal discomfort such as cramping in about 20% of patients. Excessive ...
-
OVERDOSAGESigns and Symptoms - There have been no reports of accidental overdosage. In the event of overdosage, it is expected that diarrhea and abdominal cramps would be the major symptoms. Medication ...
-
DOSAGE AND ADMINISTRATIONOral - Adult - The usual adult oral dosage is 2 to 3 tablespoonfuls (30 to 45 mL, containing 20 g to 30 g of Generlac Solution) three or four times daily. The dosage may be adjusted every day or two ...
-
HOW SUPPLIEDGenerlac Solution (Lactulose Solution, USP, 10 g/15 mL) is a colorless to yellow, unflavored solution containing 10 g of lactulose/15 mL (667 mg lactulose/mL). It is available in the following ...
-
PRINCIPAL DISPLAY PANELGenerlac Solution (Lactulose Solution, USP) 10 g/15 mL - NDC 62135-892-47 - 473 mL Bottle Label
-
INGREDIENTS AND APPEARANCEProduct Information