Label: SPECTRAMAST LC- ceftiofur hydrochloride suspension
- NDC Code(s): 54771-5279-1, 54771-5279-2
- Packager: Zoetis Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated March 26, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONSPECTRAMAST® LC - (ceftiofur intramammary suspension) Sterile Suspension - For Intramammary Infusion in Lactating Cows Only - FOR USE IN ANIMALS ONLY — NOT FOR HUMAN USE
-
CAUTIONFederal law restricts this drug to use by or on the order of a licensed veterinarian. Federal law prohibits extra-label use of this drug in lactating dairy cattle for disease prevention purposes ...
-
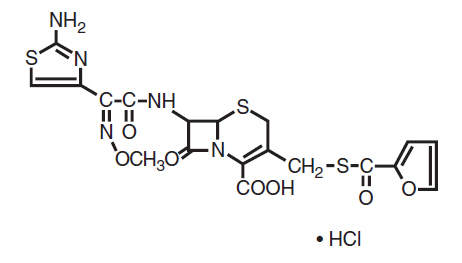
DESCRIPTIONCeftiofur hydrochloride is a cephalosporin antibiotic. Chemical Structure of Ceftiofur Hydrochloride - U-64279A - Chemical Name of Ceftiofur Hydrochloride - 5-Thia-1-azabicyclo [4.2.0 ...
-
INDICATIONS FOR USESPECTRAMAST® LC (ceftiofur intramammary suspension) Sterile Suspension is indicated for use in lactating dairy cattle for (1) the treatment of clinical mastitis associated with coagulase-negative ...
-
DOSAGEDOSAGE - Infuse one (1) syringe into each affected quarter. Repeat this treatment in 24 hours. For extended duration therapy, once daily treatment may be repeated for up to 8 consecutive days.
-
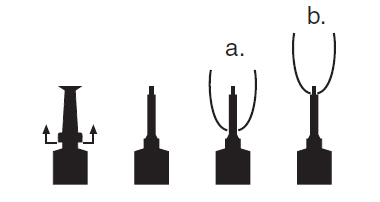
DIRECTIONS FOR USING THE PLASTET® DISPOSABLE SYRINGEThe syringe is designed to provide the choice of either insertion of the full cannula as has traditionally been practiced, or insertion of no more than 1/8 inch of the cannula, as reported by ...
-
ADMINISTRATIONTreatment: Wash teats thoroughly with warm water containing a suitable dairy antiseptic. Dry teats thoroughly. Milk out udder completely. Using an appropriate antiseptic teat wipe (containing at ...
-
CONTRAINDICATIONSAs with all drugs, the use of SPECTRAMAST® LC Sterile Suspension is contraindicated in animals previously found to be hypersensitive to the drug.
-
SPL UNCLASSIFIED SECTIONDiscard Empty Container: DO NOT REUSE - KEEP OUT OF REACH OF CHILDREN
-
WARNINGSPenicillins and cephalosporins can cause allergic reactions in sensitized individuals. Topical exposures to such antimicrobials, including ceftiofur, may elicit mild to severe allergic reactions ...
-
CONTACT INFORMATIONThe Safety Data Sheet contains more detailed occupational safety information. For a copy of the Safety Data Sheet or to report adverse reactions, call Zoetis Inc. at 1-888-963-8471. For additional ...
-
RESIDUE WARNINGS1. Milk taken from cows during treatment (a maximum of eight daily infusions) and for 72 hours after the last treatment must not be used for human consumption. 2. Following label use for up to ...
-
PRECAUTIONPRECAUTION - Following intramammary infusion with antibiotics in lactating cows, milk obtained during treatment and during the milk discard period should be properly discarded and not fed to calves ...
-
CLINICAL MICROBIOLOGYCeftiofur is a broad-spectrum cephalosporin antibiotic that exerts its effect by inhibiting bacterial cell wall synthesis. Like other s-lactam antimicrobial agents, cephalosporins inhibit cell ...
-
EFFECTIVENESSIn 1999 to 2000, the efficacy of ceftiofur was demonstrated in a pivotal multi-location field trial in lactating dairy cattle with clinical mastitis in one quarter. Ceftiofur was formulated in ...
-
ANIMAL SAFETYA pivotal GLP udder irritation study was conducted in 40 cows to assess udder irritation following daily intramammary infusion of an oil‑based suspension containing 125 mg of ceftiofur for up to 8 ...
-
MILK AND TISSUE RESIDUE DEPLETIONA metabolism study in cattle using radiolabeled ceftiofur provided the data to establish tolerances for ceftiofur-related residues (as desfuroylceftiofur) in tissue and milk. These tolerances are ...
-
STORAGE CONDITIONSStore at controlled room temperature 20° to 25° C (68° to 77° F). Protect from light. Store syringes in carton or pail until used.
-
HOW SUPPLIEDSPECTRAMAST® LC Sterile Suspension is available in cartons containing 1 unbroken package of 12–10 mL PLASTET® Disposable Syringes and in pails containing 12 unbroken packages of 12-10 mL PLASTET ...
-
NO TITLEApproved by FDA under NADA # 141-238 - zoetis - Distributed by: Zoetis Inc. Kalamazoo, MI 49007 - www.spectramast.com or call 1-888-963-8471 - Revised: December 2022 - 40040154
-
PRINCIPAL DISPLAY PANEL - 12-10 mL Syringe Carton40040155
-
INGREDIENTS AND APPEARANCEProduct Information