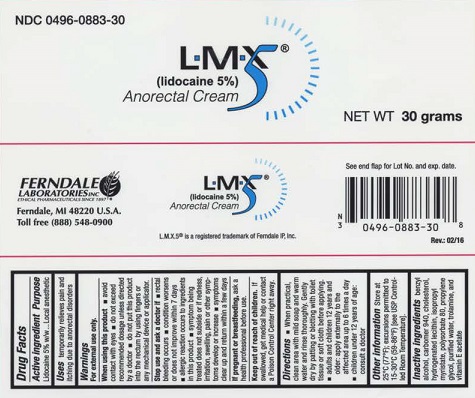
Label: LMX5- lidocaine cream
- NDC Code(s): 0496-0883-15, 0496-0883-30, 0496-0883-97
- Packager: Ferndale Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
When using this product
- avoid contact with eyes
- do not exceed recommended dosage unless directed by a doctor
- avoid contact with eyes
-
Directions
- When practical, clean area with mild soap and warm water and rinse thoroughly. Gently dry by patting or blotting with toilet tissue or soft cloth before applying.
- Adults and Children 12 years and older: Apply to the affected area up to 6 times a day.
- Children under 12 years of age: Consult a doctor.
- Other information
- Inactive Ingredients
- Package Label
-
INGREDIENTS AND APPEARANCE
LMX5
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0496-0883 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CARBOMER HOMOPOLYMER TYPE C (UNII: 4Q93RCW27E) CHOLESTEROL (UNII: 97C5T2UQ7J) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) TROLAMINE (UNII: 9O3K93S3TK) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0496-0883-30 30 g in 1 TUBE; Type 0: Not a Combination Product 10/01/2003 2 NDC:0496-0883-15 15 g in 1 TUBE; Type 0: Not a Combination Product 10/01/2003 3 NDC:0496-0883-97 1 g in 1 POUCH; Type 0: Not a Combination Product 10/01/2003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 10/01/2003 Labeler - Ferndale Laboratories, Inc. (005320536) Establishment Name Address ID/FEI Business Operations Ferndale Laboratories, Inc. 005320536 manufacture(0496-0883)